Abstract
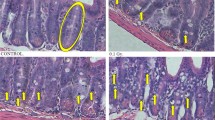
The involvement of the p53 gene in apoptosis of many cell types towards γ-radiation is well established. However, little information is available on the relationship between p53 status and cells’ ability to undergo apoptosis following exposure to fast neutrons. The aim of this study was to characterize the apoptotic pathway traveled by neutrons in mouse intestinal crypt cells. Each mouse received whole body doses of 0.25–8 Gy fast neutrons and were sacrificed 0, 4, 6, 12, 24, 48, and 72 h, respectively, after irradiation. Apoptosis of crypt cells and expression of p53, cyclin A, cyclin B, cyclin D, and cyclin E were measured. The apoptosis in crypt cells was maximal at 4 and 6 h after irradiation, showing a gradual decline at 24 h. The highest frequency of apoptosis was seen at a 1 Gy dose and then declined gradually beyond a 2 Gy dose with high levels of damage. In immunoblot analysis, apoptosis was confirmed to be dependent on p53 function after fast-neutron irradiation. In addition, cyclin B1, cyclin D, and cyclin E were overexpressed in intestinal cells after fast-neutron irradiation and their immunoreactivities were increased strongly in round and oval cells of laminar propria in villi core and crypts. The results of the current study suggest that apoptosis in crypt cells shows a time- and dose-dependent increase after fast-neutron irradiation. In addition, fast-neutron-induced apoptosis in mouse intestinal crypt cells appears to be related to the increase in functional p53 proteins to a level sufficient to initiate apoptosis and up-regulation of cell-cycle-regulated proteins, which may lead to resistance to DNA damage through cell cycle arrest, is involved deeply in protection of gastrointestinal cells after low doses of fast-neutron irradiation. (Mol Cell Biochem 270: 21–28, 2005)
Similar content being viewed by others
References
Hertveldt K, Philippe J, Thierenss H, Cornelissen M, Vral A, De Ridder L: Flow cytometry as a quantitive and sensitive method to evaluate low dose radiation induced apoptosis in vitro in human peripheral blood lymphocytes. Int J Radiat Bio 71: 429–433, 1997
Hall EJ, Roizin-Towle L, Theus RB, August L: Radiobiological properties of high energy cyclotron produced neutrons used in radiotherapy. Radiology 117:173–178, 1975
Komatsu K, Sawada S, Takeoka S, Kodama S, Okumura Y: Dose rate effects of neutrons and gamma-rays on the induction of mutation and oncogenic transformation in plateau-phase mouse m5S cells. Int J Radiat Biol 63: 469–474, 1993
Kastan MB, Radin AI, Kuerbitz SJ, Onyekwere O, Wolkow CA, Civin CI, Stone KD, Woo T, Ravindranath Y, Craig RW: Levels of p53 protein increase with maturation in human hematopoietic cells. Cancer Res 51: 4279–4286, 1991
Lee JM, Bernstein A: p53 mutations increase resistance to ionizing radiation. Proc Natl Acad Sci USA 90: 5742–5746, 1993
Aref A, Yudelev M, Mohammad R, Choudhuri R, Orton C, Al-Katib A: Neutron and photon clonogenic survival curves of two chemotherapy resistant human intermediate-grade non-Hodgkin lymphoma cell lines. Int J Radiat Oncol Biol Phys 45: 999–1003, 1999
Goodhead DT: Mechanisms for the biological effectiveness of high-LET radiations. J Radiat Res 40: 1–13, 1999
Fischer B, Coelho D, Dufour P, Bergerat JP, Denis JM, Gueulette J, Bischoff P: Caspase 8-mediated cleavage of the pro-apoptotic BCL-2 family member BID in p53-dependent apoptosis. Biochem Biophys Res Commun 306: 516–522, 2003
Britten RA, Warenius HM, White R, Browning PG, Green JA: Melphalan resistant human ovarian tumour cells are cross-resistant to photons, but not to high LET neutrons. Radiother Oncol 18: 357–363, 1990
Iliakis G: Cell cycle regulation in irradiated and nonirradiated cells. Semin Oncol 24: 602–615, 1997
Elledge SJ: Cell cycle checkpoint: Prevent an identity crisis. Science 274: 1664–1672, 1996
Waldman T, Zhang Y, Dillehay L, Yu J, Kinzler K, Vogelstein B, Williams J: Cell-cycle arrest versus cell death in cancer therapy. Nat Med 3: 1034–1036, 1997
Kim SH, Jeong KS, Ryu SY, Kim TH: Panax ginseng prevents apoptosis in hair follicles and accelerates recovery of hair medullary cells in irradiated mice. In Vivo 12: 219–222, 1998
Kerr JF, Willie AH, Currie AR: Apoptosis: A basic biological phenomenon with wide-ranging implications in the tissue kinetics. Br J Cancer 26: 239–257, 1972
Jee Y, Kim G, Tanuma N, Matsumoto Y: STAT expression and localization in the central nervous system during autoimmune encephalomyelitis in Lewis rats. J Neuroimmunol 114: 40–47, 2001
Cross SM, Sanchez CA, Morgan CA, Schimke MK, Ramel S, Idzerda RL, Raskind WH, Reid BJ: A p53-dependent mouse spindle checkpoint. Science 267: 1353–1356, 1995
Maltzman W, Czyzyk L: UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol Cell Biol 4: 1689–1694, 1984
Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Balmain A, Lane DP, Hall PA: The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res 54: 614–617, 1994
Clarke AR, Gledhill S, Hooper ML, Bird CC, Wyllie AH: p53 dependence of early apoptotic and proliferative responses within the mouse intestinal epithelium following gamma-irradiation. Oncogene 9: 1767–1773, 1994
Gottlieb E, Haffner R, King A, Asher G, Gruss P, Lonai P, Oren M: Transgenic mouse model for studying the transcriptional activity of the p53 protein: Age- and tissue-dependent changes in radiation-induced activation during embryogenesis. EMBO J 16: 1381–1390, 1997
Balcer-Kubiczek EK, Zhang XF, Harrison GH, Zhou XJ, Vigneulle RM, Ove R, McCready WA, Xu JF: Delayed expression of hpS2 and prolonged expression of CIP1/WAF1/SDI1 in human tumor cells irradiated with X-rays, fission neutron or 1 GeV/nucleon Fe ions. Int J Radiat Biol 75: 529–541, 1999
Arai T, Kida Y, Harmon BV, Gobe GC: Comparative alterations in p53 expression and apoptosis in the irradiated rat small and large intestine. Br J Cancer 74: 406–412, 1996
Jackson SP: The recognition of DNA damage. Curr Opin Genet Dev 6: 19–25, 1996
Wood RD: DNA repair in eukaryotes. Annu Rev Biochem 65: 135–167, 1996
Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW: Participation of p53 protein in the cellular response to DNA damage. Cancer Res 51: 6304–6311, 1991
Kastan MB, Zhan Q, el-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJ Jr: A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 71: 587–597, 1992
Chen X, Ko LJ, Jayaraman L, Prives C: p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev 10: 2438–2451, 1996
Lassus P, Ferlin M, Piette J, Hibner U: Anti-apoptotic activity of low levels of wild-type p53. EMBO J 15: 4566–4573, 1996
Ronen D, Schwartz D, Teitz Y, Goldfinger N, Rotter V: Induction of HL-60 cells to undergo apoptosis is determined by high levels of wild-type p53 protein whereas differentiation of the cells is mediated by lower p53 levels. Cell Growth Differ 7: 21–30, 1996
Reed JC: Double identity for proteins of the Bcl-2 family. Nature 387: 773–776, 1997
el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B: WAF1, A potential mediator of p53 tumor suppression. Cell 75: 817–825, 1993
Dulic V, Kaufmann WK, Wilson SJ, Tlsty TD, Lees E, Harper JW, Elledge SJ, Reed SI: p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell 76: 1013–1023, 1994
Selvakumaran M, Lin HK, Miyashita T, Wang HG, Krajewski S, Reed JC, Hoffman B, Liebermann D: Immediate early up-regulation of bax expression by p53 but not TGF beta 1: A paradigm for distinct apoptotic pathways. Oncogene 9: 1791–1798, 1994
Zhan Q, Fan S, Bae I, Guillouf C, Liebermann DA, O’Connor PM, Fornace AJ Jr: Induction of bax by genotoxic stress in human cells correlates with normal p53 status and apoptosis. Oncogene 9: 3743–3751, 1994
Bernhard EJ, McKenna WG, Muschel RJ: Cyclin expression and G2-phase delay after irradiation. Radiat. Res 138: S64–S67, 1994
Maity A, McKenna WG, Muschel RJ: Evidence for post-transcriptional regulation of cyclin B1 mRNA in the cell cycle and following irradiation in HeLa cells. EMBO J 14: 603–609, 1995
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jee, YH., Jeong, WI., Kim, TH. et al. p53 and cell-cycle-regulated protein expression in small intestinal cells after fast-neutron irradiation in mice. Mol Cell Biochem 270, 21–28 (2005). https://doi.org/10.1007/s11010-005-3440-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11010-005-3440-2