Abstract
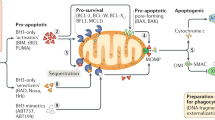
Apoptosis, or programmed cell death, plays a pivotal role in the elimination of unwanted, damaged, or infected cells in multicellular organisms and also in diverse biological processes, including development, cell differentiation, and proliferation. Apoptosis is a highly regulated form of cell death, and dysregulation of apoptosis results in pathological conditions including cancer, autoimmune and neurodegenerative diseases. The Bcl-2 family proteins are key regulators of apoptosis, which include both anti- and pro-apoptotic proteins, and a slight change in the dynamic balance of these proteins may result either in inhibition or promotion of cell death. Execution of apoptosis by various stimuli is initiated by activating either intrinsic or extrinsic pathways which lead to a series of downstream cascade of events, releasing of various apoptotic mediators from mitochondria and activation of caspases, important for the cell fate. In view of recent research advances about underlying mechanism of apoptosis, this review highlights the basics concept of apoptosis and its regulation by Bcl-2 family of protein. Furthermore, this review discusses the interplay of various apoptotic mediators and caspases to decide the fate of the cell. We expect that this review will add to the pool of basic information necessary to understand the mechanism of apoptosis which may implicate in designing better strategy to develop biomedical therapy to control apoptosis.


Similar content being viewed by others
Abbreviations
- PCD:
-
Programmed cell death
- Bcl-2:
-
B cell lymphoma-2 protein
- Bax:
-
Bcl-2 associated X protein
- Bid:
-
Bcl-2 interacting domain death agonist
- Bad:
-
Bcl-2 antagonist of cell death
- Bcl-xl:
-
Bcl-extra long
- Bim:
-
Bcl-2 interacting mediator of cell death
- Bik:
-
Bcl-2 interacting killer
- Bmf:
-
Bcl-2 modifying factor
- Boo:
-
Bcl-2 homolog of ovary
- Bcl-xs:
-
Bcl-extra short
- Bak:
-
Bcl-2 antagonistic killer
- Bok:
-
Bcl-2 related ovarian killer
- Apaf-1:
-
Apoptosis protease-activating factor-1
- Diablo:
-
Direct IAP binding Protein with low pI
- FADD:
-
Fas-associated death domain protein
- TNF-R:
-
Tumor necrosis factor receptor
- Fas-L:
-
Fas ligand
- HtrA:
-
High-temperature requirement
- IAP:
-
Inhibitor of apoptosis protein
- IMM:
-
Inner mitochondrial membrane
- Omi/HtrA2:
-
Mammalian serine protease
- SMAC:
-
Second mitochondrial activator of caspase
- TNF-α:
-
Tumor Necrosis Factor alpha
- TRADD:
-
TNF-receptor-1 associated death domain protein
- VDAC:
-
Voltage-dependent anion channel
- Cyt c:
-
Cytochrome c
- PIDDosome:
-
p53-Inducible death domain containing protein complex
- DISC:
-
Death-inducing signaling complex
References
Kerr JFR, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Adams JM (2003) Ways of dying: multiple pathways to apoptosis. Genes Dev 17:2481–2495
Saikumar P, Dong Z, Mikhailov V, Denton M, Weinberg JM, Venkatachalam MA (1999) Apoptosis: definition, mechanisms, and relevance to disease. Am J Med 107:489–506
Gulbins E, Jekle A, Ferlinz K, Grassme H, Lang F (2000) Physiology of apoptosis. Am J Physiol Renal Physiol 279:605–615
Janssen O, Qian J, Linkermann A, Kabelitz D (2003) CD95 ligand—death factor and costimulatory molecule? Cell Death Differ 10:1215–1225
Schutze S, Tchikov V, Schneider-Brachert W (2008) Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nat Rev Mol Cell Biol 9:655–662
Ashkenazi A (2002) Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer 2:420–430
Letai A (2006) Growth factor withdrawal and apoptosis: the middle game. Mol Cell 21:728–730
Zhang Y, Xing D, Liu L (2009) PUMA promotes Bax translocation by both directly interacting with Bax and by competitive binding to Bcl-X L during UV-induced apoptosis. Mol Biol Cell 20:3077–3087
Gentile M, Latonen L, Laiho M (2003) Cell cycle arrest and apoptosis provoked by UV radiation-induced DNA damage are transcriptionally highly divergent responses. Nucleic Acids Res 31:4779–4790
Stevenson MA, Pollock SS, Coleman CN, Calderwood SK (1994) X-irradiation, phorbol esters, and H2O2 stimulate mitogen-activated protein kinase activity in NIH-3T3 cells through the formation of reactive oxygen intermediates. Cancer Res 54:12–15
Dudeja V, Mujumdar N, Phillips P, Chugh R, Borja-Cacho D, Dawra RK, Vickers SM, Saluja AK (2009) Heat shock protein 70 inhibits apoptosis in cancer cells through simultaneous and independent mechanisms. Gastroenterology 136:1772–1782
Solary E, Droin N, Bettaieb A, Corcos L, Dimanche-Boitrel MT, Garrido C (2000) Positive and negative regulation of apoptotic pathways by cytotoxic agents in hematologica. Leukemia 14:1833–1849
Ahsan H, Reagan-Shaw S, Breur J, Ahmad N (2007) Sanguinarine induces apoptosis of human pancreatic carcinoma AsPC-1 and BxPC-3 cells via modulations in Bcl-2 family proteins. Cancer Lett 249:198–208
Meng SJ, Yu LJ (2010) Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci 11:1509–1526
Gu X, Song X, Dong Y, Cai H, Walters E, Zhang R, Pang X, Xie T, Guo Y, Sridhar R, Califano JA (2008) Vitamin E succinate induces ceramide-mediated apoptosis in head and neck squamous cell carcinoma in vitro and in vivo. Clin Cancer Res 14:1840–1848
Lancellotti M, Pereira RF, Cury GG, Hollanda LM (2009) Pathogenic and opportunistic respiratory bacteria-induced apoptosis. Braz J Infect Dis 13:226–231
Sorensen CM (2004) Bcl-2 family members and disease. Biochim Biophys Acta 1644:179–188
Thompson CB (1995) Apoptosis in the pathogenesis and treatment of disease. Science 267:1456–1462
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Bidere N, Su HC, Lenardo MJ (2006) Genetic disorders of programmed cell death in the immune system. Annu Rev Immunol 24:321–352
Mersich S, Gadaleta P (2003) Nuevas estrategias terapéuticas basadas en apoptosis y virus. Acta Bioquímica Clínica Latinoamericana 37:13–21
Fisher DE (1994) Apoptosis in cancer therapy: crossing the threshold. Cell 78:539–542
Johnstone RW, Ruefli AA, Lowe SW (2002) Apoptosis: a link between cancer genetics and chemotherapy. Cell 108:153–164
Pećina-Slaus N (2009) Genetic and molecular insights into apoptosis. Acta Med Croatica 63(Suppl 2):13–19
Schaffitzel E, Hertweck M (2006) Recent aging research in Caenorhabditis elegans. Exp Gerontol 41:557–563
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116(2):205–219
Horvitz HR (1999) Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Cancer Res 59:1701S–1706S
McDonnell TJ, Deane N, Platt FM, Nunez G, Jaeger U, McKearn JP, Korsmeyer SJ (1989) Bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell 57:79–88
Vaux DL, Cory S, Adams JM (1988) Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335:440–442
Vaux DL, Weissman IL, Kim SK (1992) Prevention of programmed cell death in Caenorhabditis elegans by human bcl-2. Science 258:1955–1957
Hengartner MO, Horvitz HR (1994) C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell 76:665–676
Mohamad N, Gutiérrez A, Núñez M, Cocca C, Martín G, Cricco G, Medina V, Rivera E, Bergoc R (2005) Mitochondrial apoptotic pathways. Biocell 29:149–161
Fan TJ, Han LH, Cong RS, Liang J (2005) Caspase family proteases and apoptosis. Acta Biochimica et Biophysica Sinica 37:719–727
Li J, Yuan J (2008) Caspases in apoptosis and beyond. Oncogene 27(48):6194–6206
Petros AM, Olejniczak ET, Fesik SW (2004) Structural biology of the Bcl-2 family of proteins. Biochim Biophys Acta 1644:83–94
Germain M, Shore GC (2003) Cellular distribution of Bcl-2 family proteins. Sci STKE 173:pe10
Budd R (2001) Activation-induced cell death. Curr Opin Immunol 13:356–362
Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ (1996) BID: a novel BH3 domain-only death agonist. Genes Dev 10:2859–2869
Brunelle JK, Letai A (2009) Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci 122:437–441
Puthalakath H, Strasser A (2002) Keeping fillers on a tight leash: transcriptional and post-translational control of the proapoptotic activity of BH3-only proteins. Cell Death Differ 9:505–512
Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM (1999) Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci USA 96:14943–14948
Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ (1993) Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75:229–240
Ross AJ, Waymire KG, Moss JE, Parlow AF, Skinner MK, Russell LD, MacGregor GR (1998) Testicular degeneration in Bcl-w-deficient mice. Nat Genet 18:251–256
Hamasaki A, Sendo F, Nakayama K, Ishida N, Negishi I, Nakayama K, Hatakeyama S (1998) Accelerated neutrophil apoptosis in mice lacking A1-a, a subtype of the Bcl-2-related A1 gene. J Exp Med 188:1985–1992
Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ (2000) Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev 14:23–27
Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S (1995) Massive cell death of immature hematopoietic cells and neurons in Bcl-x deficient mice. Science 267:1506–1510
Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9:47–59
Brooks C, Wei Q, Feng L, Dong G, Tao Y, Mei L, Xie ZJ, Dong Z (2007) Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci USA 104:11649–11654
Mikhailov V et al (2003) Association of Bax and Bak homooligomers in mitochondria. Bax requirement for Bak reorganization and cytochrome c release. J Biol Chem 278:5367–5376
Basañez G, Sharpe JC, Galanis J, Brandt TB, Hardwick JM, Zimmerberg J (2002) Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J Biol Chem 277:49360–49365
Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD (2002) Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111:331–342
Yethon JA, Epand RF, Leber B, Epand RM, Andrews DW (2003) Interaction with a membrane surface triggers a reversible conformational change in Bax normally associated with induction of apoptosis. J Biol Chem 278:48935–48941
Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727–730
Wang X (2001) The expanding role of mitochondria in apoptosis. Genes Dev 15:922–2933
Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, Andrews DW (2008) Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell 135(6):1074–1084
Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ (2002) Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2:183–192
Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR (2004) Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303:1010–1014
Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH (2006) Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol 8:1348–1358
Pagliari LJ, Kuwana T, Bonzon C, Newmeyer DD, Tu S, Beere HM, Green DR (2005) The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc Natl Acad Sci USA 102:17975–17980
Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, Walensky LD (2008) BAX activation is initiated at a novel interaction site. Nature 455:1076–1081
Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, Strasser A, Kluck RM, Adams JM, Huang DC (2007) Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315:856–859
Fletcher JI, Meusburger S, Hawkins CJ, Riglar DT, Lee EF, Fairlie WD, Huang DC, Adams JM (2008) Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc Natl Acad Sci USA 105:18081–18087
Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ (2003) VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301:513–517
Li H, Zhu H, Xu CJ, Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491–501
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481–490
Billen LP, Shamas-Din A, Andrews DW (2008) Bid: a Bax-like BH3 protein. Oncogene 27(Suppl 1):S93–S104
Oh KJ, Barbuto S, Pitter K, Morash J, Walensky LD, Korsmeyer SJ (2006) A membrane-targeted BID BCL-2 homology 3 peptide is sufficient for high potency activation of BAX in vitro. J Biol Chem 281:36999–37008
Eskes R, Desagher S, Antonsson B, Martinou JC (2000) Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol 20:929–935
Balakrishnan G, Hu Y, Oyerinde OF, Su J, Groves JT, Spiro TG (2007) A conformational switch to β-sheet structure in cytochrome c leads to heme exposure. Implications for cardiolipin peroxidation and apoptosis. J Am Chem Soc 129:504–505
Kim TH et al (2004) Bid–cardiolipin interaction at mitochondrial contact site contributes to mitochondrial cristae reorganization and cytochrome c release. Mol Biol Cell 15:3061–3072
Giordano A et al (2005) tBid induces alterations of mitochondrial fatty acid oxidation flux by malonyl-CoA-independent inhibition of carnitine palmitoyltransferase-1. Cell Death Differ 12:603–613
Tyurin VA et al (2007) Interactions of cardiolipin andlyso-cardiolipins with cytochrome c and tBid: conflict or assistance in apoptosis. Cell Death Differ 14:872–875
Orrenius S, Gogvadze V, Zhivotovsky B (2007) Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol 47:143–183
Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ (1996) Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell 87:619–628
Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell JE, Freeman WH (2000) Molecular cell biology, 4th edn. W. H. Freeman & Co, New York, Chapter 23–28
Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231–241
Tommasini I, Cerioni L, Palomba L, Cantoni O (2008) Prostaglandin E2 signals monocyte/macrophage survival to peroxynitrite via protein kinase A converging in bad phosphorylation with the protein kinase C alpha-dependent pathway driven by 5-hydroxyeicosatetraenoic acid. J Immunol 181:5637–5645
Grund K, Ahmadi R, Jung F, Funke V, Gdynia G, Benner A, Sykora J, Walczak H, Joos S, Felsberg J, Reifenberger G, Wiestler OD, Herold-Mende C, Roth W (2008) Troglitazone-mediated sensitization to TRAIL-induced apoptosis is regulated by proteasome-dependent degradation of FLIP and ERK1/2-dependent phosphorylation of BAD. Cancer Biol Ther 7:1982–1990
Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ (2009) {Beta}-arrestin-2 mediates anti-apoptotic signaling through regulation of BAD phosphorylation. J Biol Chem 284:8855–8865
Puthalakath H, Villunger A, O’Reilly LA, Beaumont JG, Coultas L, Cheney RE, Huang DC, Strasser A (2001) Bmf: a pro-apoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science 293:1829–1832
Lei K, Davis RJ (2003) JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci USA 100:2432–2437
Okuno S, Saito A, Hayashi T, Chan PH (2004) The c-Jun N-terminal protein kinase signaling pathway mediates Bax activation and subsequent neuronal apoptosis through interaction with Bim after transient focal cerebral ischemia. J Neurosci 24:7879–7887
Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Köntgen F, Adams JM, Strasser A (1999) Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286:1735–1738
Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A (2002) BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 415:922–926
Putcha GV, Moulder KL, Golden JP, Bouillet P, Adams JA, Strasser A, Johnson EM (2001) Induction of Bim, a proapoptotic BH3- only Bcl-2 family member, is critical for neuronal apoptosis. Neuron 29:615–628
Zhang L, Xing D, Chen M (2008) Bim(L) displacing Bcl-x(L) promotes Bax translocation during TNFalpha-induced apoptosis. Apoptosis 13:950–958
Wang X, Xing D, Liu L, Chen WR (2009) BimL directly neutralizes Bcl-xL to promote Bax activation during UV-induced apoptosis. FEBS Lett 583:1873–1879
Zhang Y, Adachi M, Kawamura R, Zou HC, Imai K, Hareyama M, Shinomura Y (2006) Bmf contributes to histone deacetylase inhibitor-mediated enhancing effects on apoptosis after ionizing radiation. Apoptosis 11:1349–1357
Zhang Y, Adachi M, Kawamura R, Imai K (2006) Bmf is a possible mediator in histone deacetylase inhibitors FK228 and CBHA-induced apoptosis. Cell Death Differ 13:129–140
Ramjaun AR, Tomlinson S, Eddaoudi A, Downward J (2007) Upregulation of two BH3-only proteins, Bmf and Bim, during TGF beta-induced apoptosis. Oncogene 26:970–981
Yakovlev AG, Giovanni SD, Wang G et al (2004) Bok and Noxa are essential mediators of p53-dependent apoptosis. J Biol Chem 279:28367–28374
Oda E, Ohki R, Murasawa H et al (2000) Noxa, a BH3- only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288:1053–1058
Villunger A, Michalak EM, Coultas L et al (2003) p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science 302:1036–1038
Karst AM, Li G (2007) BH3-only proteins in tumorigenesis and malignant melanoma. Cell Mol Life Sci 64:318–330
Ming L, Wang P, Bank A et al (2006) Puma dissociates Bax and Bcl-XL to induce apoptosis in colon cancer cells. J Biol Chem 281:16034–16042
Wyttenbach A, Tolkovsky AM (2006) The BH3-only protein Puma is both necessary and sufficient for neuronal apoptosis induced by DNA damage in sympathetic neurons. J Neurochem 96:1213–1226
Jabbour AM, Heraud JE, Daunt CP, Kaufmann T, Sandow J, O’Reilly LA, Callus BA, Lopez A, Strasser A, Vaux DL, Ekert PG (2009) Puma indirectly activates Bax to cause apoptosis in the absence of Bid or Bim. Cell Death Differ 16:555–563
Liu Z, Lu H, Shi H et al (2005) Puma overexpression induces reactive oxygen species generation and proteasome- mediated stathmin degradation in colorectal cancer cells. Cancer Res 65:1647–1654
Hemann MT, Zilfou JT, Zhao Z et al (2004) Suppression of tumorigenesis by the p53 target Puma. Proc Natl Acad Sci USA 101:9333–9338
Adams JM, Cory S (2007) The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26:1324–1337
Riedl SJ, Salvesen GS (2007) The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol 8:405–413
Strasser A, O’Connor L, Dixit VM (2000) Apoptosis signaling. Annu Rev Biochem 69:217–245
Strasser A, Harris AW, Huang DCS, Krammer PH, Cory S (1995) Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J 14:6136–6147
Li P et al (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479–489
Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ, Los M (2009) Apoptosis and cancer: mutations within caspase genes. J Med Genet 46:497–510
Locksley RM, Killeen N, Lenardo MJ (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487–501
Wajant H (2002) The Fas signaling pathway: more than a paradigm. Science 296:1635–1636
Chen G, Goeddel D (2002) TNF-1 signaling: a beautiful pathway. Science 296:1634–1635
Yang JK, Wang L, Zheng L, Wan F, Ahmed M, Lenardo MJ, Wu H (2005) Crystal structure of MC159 reveals molecular mechanism of DISC assembly and FLIP inhibition. Mol Cell 20:939–949
Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH (2000) Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ 7:1166–1173
Lartigue L, Kushnareva Y, Seong Y, Lin H, Faustin B, Newmeyer DD (2009) Caspase-independent mitochondrial cell death results from loss of respiration, not cytotoxic protein release. Mol Biol Cell 20:4871–4884
Pellegrini L, Scorrano L (2007) A cut short to death: Parl and Opa1 in the regulation of mitochondrial morphology and apoptosis. Cell Death Differ 14:1275–1284
Liu X, Kim CN, Yang J, Jemmerson R, Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147–157
Li F et al (1997) Cell-specific induction of apoptosis by microinjection of cytochrome c. Bcl-xL has activity independent of cytochrome c release. J Biol Chem 272:30299–30305
Zhivotovsky B, Orrenius S, Brustugun OT, Doskeland SO (1998) Injected cytochrome c induces apoptosis. Nature 391:449–450
Newmeyer DD, Farschon DM, Reed JC (1994) Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell 79:353–364
Kluck RM, Bossy-Wetzel E, Green DR, New meyer DD (1997) The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275:1132–1136
Glazunova VA, Shtil AA (2008) Mitochondrial mechanisms of apoptosis in response to DNA damage. Mol Biol (Mosk) 42:765–771
Ravi D, Das KC (2004) Redox-cycling of anthracyclines by thioredoxin system: increased superoxide generation and DNA damage. Cancer Chemother Pharmacol 54:449–458
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X (1997) Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275:1129–1132
Bajt ML, Cover C, Lemasters JJ, Jaeschke H (2006) Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci 94:217–225
Li LY, Luo X, Wang X (2001) Endonuclease G (EndoG) is an apoptotic DNAse when released from mitochondria. Nature 412:95–99
Zhang J, Liu X, Scherer DC, van Kaer L, Wang X, Xu M (1998) Resistance to DNA fragmentation and chromatin condensation in mice lacking the DNA fragmentation factor. Proc Natl Acad Sci 95:12480–12485
Ohsato T, Ishihara N, Muta T, Umeda S, Ikeda S, Mihara K, Hamasaki N, Kang D (2002) Mammalian mitochondrial endonuclease G digestion of R-loops and localization in intermembrane space. Eur J Biochem 269:5765–5770
van Loo G, Schotte P, van Gurp M, Demol H, Hoorelbeke B, Gevaert K, Rodriguez I, Ruiz-Carrillo A, Vandekerckhove J, Declercq W, Beyaert R, Vandenabeele P (2001) Endonuclease G: a mitochondrial protein released in apoptosis and involved in caspase-independent DNA degradation. Cell Death Differ 8:1136–1142
Widlak P, Li LY, Wang X, Garrard WT (2001) Action of recombinant human apoptotic endonuclease G on naked DNA and chromatin substrates: cooperation with exonuclease and DNase. I. J Biol Chem 276:48404–48409
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G (1999) Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397:441–446
Kondo K, Obitsu S, Ohta S, Matsunami K, Otsuka H, Teshima R (2010) Poly(ADP-ribose) polymerase (PARP)-1-independent apoptosis-inducing factor (AIF) release and cell death are induced by eleostearic acid and blocked by α-tocopherol and MEK inhibition. J Biol Chem 285:13079–13091
Miramar MD, Costantini P, Ravagnan L, Saraiva LM, Haouzi D, Brothers G, Penninger JM, Peleato ML, Kroemer G, Susin SA (2001) NADH oxidase activity of mitochondrial apoptosis-inducing factor. J Biol Chem 276:16391–16398
Joza N, Pospisilik JA, Hangen E, Hanada T, Modjtahedi N, Penninger JM, Kroemer G (2009) AIF: not just an apoptosis-inducing factor. Ann N Y Acad Sci 1171:2–11
Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, Ferri KF, Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong YY, Mak TW, Zuniga-Pflucker JC, Kroemer G, Penninger JM (2001) Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410:549–554
Schulthess FT, Katz S, Ardestani A, Kawahira H, Georgia S, Bosco D, Bhushan A, Maedler K (2009) Deletion of the mitochondrial flavoprotein apoptosis inducing factor (AIF) induces β-cell apoptosis and impairs β-cell mass. PLoS One 4:4394
Cande C, Cecconi F, Dessen P, Kroemer G (2002) Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci 115:4727–4734
Yu W, Gubkina O, Mechawar N, Elwell D, Quirion R, Krantic S (2009) Expression of apoptosis-inducing factor (AIF) in the aged rat brain. Neurobiol Aging 32(1):179–180
Susin SA, Daugas E, Ravagnan L, Samejima K, Zamzami N, Loeffler M, Costantini P, Ferri KF, Irinopoulou T, Prevost MC, Brothers G, Mak TW, Penninger J, Earnshaw WC, Kroemer G (2000) Two distinct pathways leading to nuclear apoptosis. J Exp Med 192:571–580
Pallast S, Arai K, Pekcec A, Yigitkanli K, Yu Z, Wang X, Lo EH, Leyen KV (2010) Increased nuclear apoptosis-inducing factor after transient focal ischemia: a 12/15-lipoxygenase-dependent organelle damage pathway. J Cereb Blood Flow Metab 30:1157–1167
Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL (2000) Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102:43–53
Kominsky DJ, Bickel RJ, Tyler KL (2002) Reovirus-induced apoptosis requires mitochondrial release of Smac/DIABLO and involves reduction of cellular inhibitor of apoptosis protein levels. J Virol 76:11414–11424
Wilkinson JC, Wilkinson AS, Scott FL, Csomos RA, Salvesen GS, Duckett CS (2004) Neutralization of Smac/Diablo by inhibitors of apoptosis (IAPs). A caspase-independent mechanism for apoptotic inhibition. J Biol Chem 279:51082–51090
Martinez-Ruiz G, Maldonado V, Ceballos-Cancino G, Grajeda JP, Melendez-Zajgla J (2008) Role of Smac/DIABLO in cancer progression. J Exp Clin Cancer Res 26:48
Arellano-Llamas A, Garcia FJ, Perez D, Cantu D, Espinosa M, De la Garza JG, Maldonado V, Melendez-Zajgla J (2006) High Smac/DIABLO expression is associated with early local recurrence of cervical cancer. BMC Cancer 6:256
Kohli M, Yu J, Seaman C, Bardelli A, Kinzler KW, Vogelstein B, Lengauer C, Zhang L (2004) SMAC/Diablo-dependent apoptosis induced by nonsteroidal antiinflammatory drugs (NSAIDs) in colon cancer cells. Proc Natl Acad Sci USA 101:16897–16902
Augello C, Caruso L, Maggioni M, Donadon M, Montorsi M, Santambrogio R, Torzilli G, Vaira V, Pellegrini C, Roncalli M, Coggi G, Bosari S (2009) Inhibitors of apoptosis proteins (IAPs) expression and their prognostic significance in hepatocellular carcinoma. BMC Cancer 9:125
Gray CW, Ward RV, Karran E, Turconi S, Rowles A, Viglienghi D, Southan C, Barton A, Fantom KG, West A, Savopoulos J, Hassan NJ, Clinkenbeard H, Hanning C, Amegadzie B, Davis JB, Dingwall C, Livi GP, Creasy CL (2000) Characterization of human HtrA2, a novel serine protease involved in the mammalian cellular stress response. Eur J Biochem 267:5699–5710
Suzuki Y, Imai Y, Nakayama H, Takahashi K, Takio K, Takahashi R (2001) A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol Cell 8:613–621
Verhagen AM, Silke J, Ekert PG, Pakusch M, Kaufmann H, Connolly LM, Day CL, Tikoo A, Burke R, Wrobel C, Moritz RL, Simpson RJ, Vaux DL (2002) HtrA2 promotes cell death through its serine protease activity and its ability to antagonize inhibitor of apoptosis proteins. J Biol Chem 277:445–454
Martins LM, Iaccarino I, Tenev T, Gschmeissner S, Totty NF, Lemoine NR, Savopoulos J, Gray CW, Creasy CL, Dingwall C, Downward J (2002) The serine protease Omi/HtrA2 regulates apoptosis by binding XIAP through a repear-like motif. J Biol Chem 277:439–444
Martins LM, Morrison A, Klupsch K, Fedele V, Moisoi N, Teismann P, Abuin A, Grau E, Geppert M, Livi GP, Creasy CL, Martin A, Hargreaves I, Heales SJ, Okada H, Brandner S, Schulz JB, Mak T, Downward J (2004) Neuroprotective role of the reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol Cell Biol 24:9848–9862
Balakrishnan MP, Cilenti L, Mashak Z, Popat P, Alnemri ES, Zervos AS (2009) THAP5 is a human cardiac-specific inhibitor of cell cycle that is cleaved by the proapoptotic Omi/HtrA2 protease during cell death. Am J Physiol Heart Circ Physiol 297:H643–H653
Pruefer FG, Lizarraga F, Maldonado V, Melendez-Zajgla J (2008) Participation of Omi Htra2 serine-protease activity in the apoptosis induced by cisplatin on SW480 colon cancer cells. J Chemother 20:348–354
Ding X, Patel M, Shen D, Herzlich AA, Cao X, Villasmil R, Klupsch K, Tuo J, Downward J, Chan CC (2009) Enhanced HtrA2/Omi expression in oxidative injury to retinal pigment epithelial cells and murine models of neurodegeneration. Invest Ophthalmol Vis Sci 50:4957–4966
Trencia A, Fiory F, Maitan MA, Vito P, Barbagallo AP, Perfetti A, Miele C, Ungaro P, Oriente F, Cilenti L, Zervos AS, Formisano P, Beguinot F (2004) Omi/HtrA2 promotes cell death by binding and degrading the anti-apoptotic protein ped/pea-15. J Biol Chem 279:46566–46572
Hu XY, Chen XC, Zhu ZH, Chen CH, Zeng FQ, Lu GC (2006) Effects of Omi/HtrA2 on expression of anti-apoptotic protein PED/PEA-15 and apoptosis of prostate cancer cell line PC-3. Ai Zheng 25:677–682
Krick S, Shi S, Ju W, Faul C, Tsai SY, Mundel P, Bottinger EP (2008) Mpv17l protects against mitochondrial oxidative stress and apoptosis by activation of Omi/HtrA2 protease. Proc Natl Aca. Sci USA 105:14106–14111
Salvesen GS, Duckett CS (2002) IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol 3:401–410
Vucic D, Fairbrother WJ (2007) The inhibitor of apoptosis proteins as therapeutic targets in cancer. Clin Cancer Res 13:5995–6000
Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y, Alnemri ES (2001) A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature 410:112–116
Srinivasula SM, Gupta S, Datta P, Zhang Z, Hegde R, Cheong N, Fernandes-Alnemri T, Alnemri ES (2003) Inhibitor of apoptosis proteins are substrates for the mitochondrial serine protease Omi/HtrA2. J Biol Chem 278:31469–31472
Huang Y, Park YC, Rich RL, Segal D, Myszka DG, Wu H (2001) Structural basis of caspase inhibition by XIAP: differential roles of the linker versus the BIR domain. Cell 104:81–90
Eckelman BP, Salvesen GS (2006) The human antiapoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J Biol Chem 281:3254–3260
Zobel K, Wang L, Varfolomeev E, Franklin MC, Elliott LO, Wallweber HJ, Okawa DC, Flygare JA, Vucic D, Fairbrother WJ, Deshayes K (2006) Design, synthesis, and biological activity of apotent Smac mimetic that sensitizes cancer cells to apoptosis by antagonizing IAPs. ACSChemBiol 1:525–533
Kempkensteffen C, Hinz S, Christoph F, Krause H, Magheli A, Schrader M, Schostak M, Miller K, Weikert S (2008) Expression levels of the mitochondrial IAP antagonists Smac/DIABLO and Omi/HtrA2 in clear-cell renal cell carcinomas and their prognostic value. J Cancer Res Clin Oncol 134:543–550
Wagener N, Crnković-Mertens I, Vetter C, Macher-Göppinger S, Bedke J, Gröne EF, Zentgraf H, Pritsch M, Hoppe-Seyler K, Buse S, Haferkamp A, Autschbach F, Hohenfellner M, Hoppe-Seyler F (2007) Expression of inhibitor of apoptosis protein Livin in renal cell carcinoma and non-tumorous adult kidney. Br J Cancer 97:1271–1276
Fulda S (2008) Targeting inhibitor of apoptosis proteins (IAPs) for cancer therapy. Anticancer Agents Med Chem 8:533–539
Wang J, Lenardo MJ (2000) Roles of caspases in apoptosis, development, and cytokine maturation revealed by homozygous gene deficiencies. J Cell Sci 113(Pt 5):753–757
Shi Y (2002) Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 9:459–470
Los M, van de Craen M, Penning CL, Schenk H, Westendorp M, Baeuerle PA, Droge W, Krammer PH, Fiers W, Schulze-Osthoff K (1995) Requirement of an ICE/Ced-3 protease for Fas/Apo-1–1mediated apoptosis. Nature 375:81–83
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281:1312–1316
Sprick MR and Walczak H (2004) The interplay between the Bcl-2 family and death receptor-mediated apoptosis. Biochim Biophys Acta 1644:125–132
Bonzon C, Bouchier-Hayes L, Pagliari LJ, Green DR, Newmeyer DD (2006) Caspase-2-induced apoptosis requires bid cleavage: a physiological role for bid in heat shock-induced death. Mol Biol Cell 17:2150–2157
Shi Y (2008) Apoptosome assembly Methods. Enzymol 442:141–156
Bao Q, Shi Y (2007) Apoptosome: a platform for the activation of initiator caspases. Cell Death Differ 14:56–65
Ow YP, Green DR, Hao Z, Mak TW (2008) Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol 9:532–542
Peter ME, Krammer PH (2003) The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ 10:26–35
Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, peter ME (1995) Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J 14:5579–5588
Yan N, Shi Y (2005) Mechanisms of apoptosis through structural biology. Annu Rev Cell Dev Biol 21:35–56
Donepudi M, Mac Sweeney A, Briand C, Grutter MG (2003) Insights into the regulatory mechanism for caspase-8 activation. Mol Cell 11:543–549
Davis AR, Lotocki G, Marcillo AE, Dietrich WD, Keane RW (2007) FasL, Fas, and death-inducing signaling complex (DISC) proteins are recruited to membrane rafts after spinal cord injury. J Neurotrauma 24:823–834
Boatright KM, Deis C, Denault JB, Sutherlin DP, Salvesen GS (2004) Activation of caspases-8 and -10 by FLIP(L). Biochem J 382:651–657
O’Reilly LA, Ekert P, Harvey N et al (2002) Caspase-2 is not required for thymocyte or neuronal apoptosis even thoughcleavage of caspase-2 is dependent on both Apaf-1 and caspase 9. Cell Death Differ 9:832–841
Kim IR, Murakami K, Chen NJ, Saibil SD, Matysiak-Zablocki E, Elford AR, Bonnard M, Benchimol S, Jurisicova A, Yeh WC, Ohashi PS (2009) DNA damage- and stress-induced apoptosis occurs independently of PIDD. Apoptosis 14:1039–1049
Ho LH, Read SH, Dorstyn L, Lambrusco L, Kumar S (2008) Caspase-2 is required for cell death induced by cytoskeletal disruption. Oncogene 27:3393–3404
Park MS, Kim BS, Devarajan P (2007) Hypoxia/reoxygenation injury induces apoptosis of LLC-PK1 cells by activation of caspase-2. Pediatr Nephrol 22:202–208
Gogvadze V, Orrenius S, Zhivotovsky B (2006) Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta 1757:639–647
Baptiste-Okoh N, Barsotti AM, Prives C (2008) A role for caspase 2 and PIDD in the process of p53-mediated apoptosis. Proc Natl Acad Sci USA 105:1937–1942
Comelli M, Genero N, Mavelli I (2009) Caspase-independent apoptosis in Friend’s erythroleukemia cells: role of mitochondrial ATP synthesis impairment in relocation of apoptosis-inducing factor and endonuclease G. J Bioenerg Biomembr 41:49–59
Parcellier A, Gurbuxani S, Schmitt E, Solary E, Garrido C (2003) Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem Biophys Res Commun 304:505–512
Laudanski K, Wyczechowska D (2006) The distinctive role of small heat shock proteins in oncogenesis. Arch Immunol Ther Exp (Warsz) 54:103–111
Garrido C, Schmitt E, Candé C, Vahsen N, Parcellier A, Kroemer G (2003) HSP27 and HSP70: potentially oncogenic apoptosis inhibitors. Cell Cycle 2:579–584
Lin CY, Wu HY, Wang PL, Yuan CJ (2010) Mammalian Ste20-like protein kinase 3 induces a caspase-independent apoptotic pathway. Int J Biochem Cell Biol 42:98–105
Strauss G, Westhoff MA, Fischer-Posovszky P, Fulda S, Schanbacher M, Eckhoff SM, Stahnke K, Vahsen N, Kroemer G, Debatin KM (2008) 4-hydroperoxy-cyclophosphamide mediates caspase-independent T-cell apoptosis involving oxidative stress-induced nuclear relocation of mitochondrial apoptogenic factors AIF and EndoG. Cell Death Differ 15:332–343
Satou T, Cummings BJ, Cotman CW (1995) Immunoreactivity for Bcl-2 protein within neurons in the Alzheimer’s disease brain increases with disease severity. Brain Re 697:35–43
Jarskog LF, Gilmore JH (2000) Developmental expression of Bcl-2 protein in human cortex. Brain Res Dev Brain Res 119:225–230
Levy OA, Malagelada C, Greene LA (2009) Cell death pathways in Parkinson’s disease: proximal triggers, distal effectors, and final steps. Apoptosis 14:478–500
Morissette MR, Rosenzweig A (2005) Targeting survival signaling in heart failure. Curr Opin Pharmacol 5:165–170
Donath MY, Halban PA (2004) Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia 47:581–589
Kern TS, Du Y, Miller CM, Hatala DA, Levin LA (2010) Overexpression of Bcl-2 in vascular endothelium inhibits the microvascular lesions of diabetic retinopathy. Am J Pathol 176:2550–2558
Littlewood TD, Bennett MR (2003) Apoptotic cell death in atherosclerosis. Curr Opin Lipidol 14:469–475
Susnow N, Zeng L, Margineantu D, Hockenbery DM (2009) Bcl-2 family proteins as regulators of oxidative stress. Semin Cancer Biol 9:42–49
Hajra KM, Liu JR (2004) Apoptosome dysfunction in human cancer. Apoptosis 9:691–704
Yip KW, Reed JC (2008) Bcl-2 family proteins and cancer. Oncogene 27:6398–6406
Reagan-Shaw S, Nihal M, Ahsan H, Mukhtar H, Ahmad N (2008) Combination of vitamin E and delenium causes an induction of apoptosis of human prostate cancer cells by enhancing the Bax/Bcl-2 ration. Prostate 68:1624–1634
Cartron PF, Oliver L, Martin S, Moreau C, LeCabellec MT, Jezequel P, Meflah K, Vallette FM (2002) The expression of a new variant of the pro-apoptotic molecule Bax, Baxψ, is correlated with an increased survival of glioblastoma multiforme patients. Hum Mol Genet 11:675–687
Kirkin V, Joos S, Zornig M (2004) The role of Bcl-2 family members in tumorigenesis. Biochim Biophys Acta 1644:229–249
O’Neill J, Manion M, Schwartz P, Hockenbery DM (2004) Promises and challenges of targeting Bcl-2 anti-apoptotic proteins for cancer therapy. Biochim Biophys Acta 1705:43–51
Juin P, Geneste O, Raimbaud E, Hickman JA (2004) Shooting at survivors: Bcl-2 family members as drug targets for cancer. Biochim Biophys Acta 1644:251–260
Frankel SR (2003) Oblimersen sodium (G3139 Bcl-2 antisense oligonucleotide) therapy in Waldenstrom’s macroglobulinemia: a targeted approach to enhance apoptosis. Semin Oncol 30:300–304
Degterev A, Lugovskoy A, Cardone M, Mulley B, Wagner G, Mitchison T, Yuan J (2001) Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nat Cell Biol 3:173–182
Tzung SP, Kim KM, Basañez G, Giedt CD, Simon J, Zimmerberg J, Zhang KY, Hockenbery DM (2001) Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nat Cell Biol 3:183–191
Enyedy IJ, Ling Y, Nacro K, Tomita Y, Wu X, Cao Y, Guo R, Li B, Zhu X, Huang Y, Long YQ, Roller PP, Yang D, Wang S (2001) Discovery of small-molecule inhibitors of Bcl-2 through structure-based computer screening. J Med Chem 44:4313–4324
Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M (2003) Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem 46:4259–4264
Becattini B, Kitada S, Leone M, Monosov E, Chandler S, Zhai D, Kipps TJ, Reed JC, Pellecchia M (2004) Rational design and real time, in-cell detection of the proapoptotic activity of a novel compound targeting Bcl-X(L). Chem Biol 11:389–395
Li J, Viallet J, Haura EB (2008) A small molecule pan-Bcl-2 family inhibitor, GX15–070, induces apoptosis and enhances cisplatin-induced apoptosis in non-small cell lung cancer cells. Cancer Chemother Pharmacol 61:525–534
McGregor N, Patel L, Craig M, Weidner S, Wang S, Pienta KJ (2010) AT-101 (R-(-)-gossypol acetic acid) enhances the effectiveness of androgen deprivation therapy in the VCaP prostate cancer model. J Cell Biochem 110:1187–1194
Ferrer P, Asensi M, Priego S, Benlloch M, Mena S, Ortega A, Obrador E, Esteve JM, Estrela JM (2007) Nitric oxide mediates natural polyphenol-induced Bcl-2 down-regulation and activation of cell death in metastatic B16 melanoma. J Biol Chem 282:2880–2890
Muilenburg DJ, Coates JM, Virudachalam S, Bold RJ (2010) Targeting Bcl-2-mediated cell death as a novel therapy in pancreatic cancer. J Surg Res 163(2):276–281
An J, Chen Y, Huang Z (2004) Critical upstream signals of cytochrome C release induced by a novel Bcl-2 inhibitor. J Biol Chem 279:19133–19140
Wang JL, Zhang ZJ, Choksi S, Shan S, Lu Z, Croce CM, Alnemri ES, Korngold R, Huang Z (2000) Cell permeable Bcl-2 binding peptides: a chemical approach to apoptosis induction in tumor cells. Cancer Res 60:1498–1502
Yin H, Lee GI, Sedey KA, Kutzki O, Park HS, Orner BP, Ernst JT, Wang HG, Sebti SM, Hamilton AD (2005) Terphenyl-Based Bak BH3 alpha-helical proteomimetics as low-molecular-weight antagonists of Bcl-xL. J Am Chem Soc 127:10191–10196
Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ (2004) Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science 305:1466–1470
Bombrun A, Gerber P, Casi G, Terradillos O, Antonsson B, Halazy S (2003) 3, 6-dibromocarbazole piperazine derivatives of 2-propanol as first inhibitors of cytochrome c release via Bax channel modulation. J Med Chem 46:4365–4368
Becattini B, Sareth S, Zhai D, Crowell KJ, Leone M, Reed JC, Pellecchia M (2004) Targeting apoptosis via chemical design: inhibition of bid-induced cell death by small organic molecules. Chem Biol 11:1107–1117
Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC (2003) Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature 423:456–461
Sawada M, Sun W, Hayes P, Leskov K, Boothman DA, Matsuyama S (2003) Ku70 suppresses the apoptotic translocation of Bax to mitochondria. Nat Cell Biol 5:320–329
Foster FM, Owens TW, Tanianis-Hughes J, Clarke RB, Brennan K, Bundred NJ, Streuli CH (2009) Targeting inhibitor of apoptosis proteins in combination with ErbB antagonists in breast cancer. Breast Cancer Res 1:R41
Liston P, Fong WG, Korneluk RG (2003) The inhibitors of apoptosis: there is more to life than Bcl2. Oncogene 22:8568–8580
LaCasse EC, Cherton-Horvat GG, Hewitt KE, Jerome LJ, Morris SJ, Kandimalla ER, Yu D, Wang H, Wang W, Zhang R, Agrawal S, Gillard JW, Durkin JP (2006) Preclinical characterization of AEG35156/GEM 640, a second-generation antisense oligonucleotide targeting X-linked inhibitor of apoptosis. Clin Cancer Res 12:5231–5241
Wu TY, Wagner KW, Bursulaya B, Schultz PG, Deveraux QL (2003) Development and characterization of nonpeptidic small molecule inhibitors of the XIAP/caspase-3 interaction. Chem Biol 10:759–767
Schimmer AD, Welsh K, Pinilla C, Wang Z, Krajewska M, Bonneau MJ, Pedersen IM, Kitada S, Scott FL, Bailly-Maitre B, Glinsky G, Scudiero D, Sausville E, Salvesen G, Nefzi A, Ostresh JM, Houghten RA, Reed JC (2004) Small-molecule antagonists of apoptosis suppressor XIAP exhibit broad antitumor activity. Cancer Cell 5:25–35
Chen J, Nikolovska-Coleska Z, Wang G, Qiu S, Wang S (2006) Design, synthesis, and characterization of new embelin derivatives as potent inhibitors of X-linked inhibitor of apoptosis protein. Bioorg Med Chem Lett 16:5805–5808
Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG (2004) A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science 305:1471–1474
Zobel K, Wang L, Varfolomeev E, Franklin MC, Elliott LO, Wallweber HJ, Okawa DC, Flygare JA, Vucic D, Fairbrother WJ, Deshayes K (2006) Design, synthesis, and biological activity of a potent Smac mimetic that sensitizes cancer cells to apoptosis by antagonizing IAPs. ACS Chem Biol 1:525–533
Oost TK, Sun C, Armstrong RC, Al-Assaad AS, Betz SF, Deckwerth TL, Ding H, Elmore SW, Meadows RP, Olejniczak ET, Oleksijew A, Oltersdorf T, Rosenberg SH, Shoemaker AR, Tomaselli KJ, Zou H, Fesik SW (2004) Discovery of potent antagonists of the antiapoptotic protein XIAP for the treatment of cancer. J Med Chem 47:4417–4426
Lu J, Bai L, Sun H, Nikolovska-Coleska Z, McEachern D, Qiu S, Miller RS, Yi H, Shangary S, Sun Y, Meagher JL, Stuckey JA, Wang S (2008) SM-164: a novel, bivalent Smac mimetic that induces apoptosis and tumor regression by concurrent removal of the blockade of cIAP-1/2 and XIAP. Cancer Res 68:9384–9393
Chauhan D, Neri P, Velankar M, Podar K, Hideshima T, Fulciniti M, Tassone P, Raje N, Mitsiades C, Mitsiades N, Richardson P, Zawel L, Tran M, Munshi N, Anderson KC (2007) Targeting mitochondrial factor Smac/DIABLO as therapy for multiple myeloma (MM). Blood 109:1220–1227
Arnt CR, Chiorean MV, Heldebrant MP, Gores GJ, Kaufmann SH (2002) Synthetic Smac/DIABLO peptides enhance the effects of chemotherapeutic agents by binding XIAP and cIAP1 in situ. J Biol Chem 277:44236–44243
Acknowledgments
MSO and MN thank Medical Research Chair in Ophthalmology funded by Dr. Nasser Al-Rasheed, College of Medicine, Kind Saud University for support. HA would like to thank Dr. Nihal Ahmad, School of Medicine and Public Health, University of Wiscosnsin, Madison for a cancer cell biology research fellowship. The authors would also like to thank Ms. Crisalis Longanilla-Bautista and Mr. Miaraj Siddiquei in helping with figures and proof reading.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ola, M.S., Nawaz, M. & Ahsan, H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem 351, 41–58 (2011). https://doi.org/10.1007/s11010-010-0709-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-010-0709-x