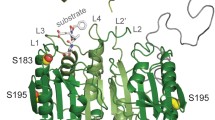
Abstract
Apoptosis, or programmed cell death, is a vital cellular process often impaired in diseases such as cancer. Aspartic acid-directed proteases known as caspases cleave a broad spectrum of cellular proteins and are central constituents of the apoptotic machinery. Caspases are regulated by a variety of mechanisms including protein phosphorylation. One intriguing mechanism by which protein kinases can modulate caspase pathways is by blocking substrate cleavage through phosphorylation of residues adjacent to caspase cleavage sites. To explore this mechanism in detail, we recently undertook a systematic investigation using a combination of bioinformatics, peptide arrays, and peptide cleavage assays to identify proteins with overlapping protein kinase and caspase recognition motifs (Duncan et al., Sci Signal 4:ra30, 2011). These studies implicated protein kinase CK2 as a global regulator of apoptotic pathways. In this article, we extend the analysis of proteins with overlapping CK2 and caspase consensus motifs to examine the convergence of CK2 with specific caspases and to identify CK2/caspase substrates known to be phosphorylated or cleaved in cells. Given its constitutive activity and elevated expression in cancer, these observations suggest that the ability of CK2 to modulate caspase pathways may contribute to a role in promoting cancer cell survival and raise interesting prospects for therapeutic targeting of CK2.



Similar content being viewed by others
References
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Cohen GM (1997) Caspases: the executioners of apoptosis. Biochem J 326(Pt 1):1–16
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281:1312–1316
Pop C, Salvesen GS (2009) Human caspases: activation, specificity, and regulation. J Biol Chem 284:21777–21781
Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS (2003) A unified model for apical caspase activation. Mol Cell 11:529–541
Cory S, Adams JM (2002) The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2:647–656
Kitazumi I, Tsukahara M (2011) Regulation of DNA fragmentation: the role of caspases and phosphorylation. FEBS J 278:427–441
Kurokawa M, Kornbluth S (2009) Caspases and kinases in a death grip. Cell 138:838–854
Duncan JS, Turowec JP, Vilk G, Li SS, Gloor GB, Litchfield DW (2010) Regulation of cell proliferation and survival: convergence of protein kinases and caspases. Biochim Biophys Acta 1804:505–510
Tozser J, Bagossi P, Zahuczky G, Specht SI, Majerova E, Copeland TD (2003) Effect of caspase cleavage-site phosphorylation on proteolysis. Biochem J 372:137–143
Duncan JS, Turowec JP, Duncan KE, Vilk G, Wu C, Luscher B, Li SS, Gloor GB, Litchfield DW (2011) A peptide-based target screen implicates the protein kinase CK2 in the global regulation of caspase signaling. Sci Signal 4(172):ra30
Shin S, Lee Y, Kim W, Ko H, Choi H, Kim K (2005) Caspase-2 primes cancer cells for TRAIL-mediated apoptosis by processing procaspase-8. EMBO J 24:3532–3542
McDonnell MA, Abedin MJ, Melendez M, Platikanova TN, Ecklund JR, Ahmed K, Kelekar A (2008) Phosphorylation of murine caspase-9 by the protein kinase casein kinase 2 regulates its cleavage by caspase-8. J Biol Chem 283:20149–20158
Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, Journot L, Antonsson B, Martinou JC (2001) Phosphorylation of bid by casein kinases I and II regulates its cleavage by caspase 8. Mol Cell 8:601–611
Yin X, Gu S, Jiang JX (2001) The development-associated cleavage of lens connexin 45.6 by caspase-3-like protease is regulated by casein kinase II-mediated phosphorylation. J Biol Chem 276:34567–34572
Torres J, Rodriguez J, Myers MP, Valiente M, Graves JD, Tonks NK, Pulido R (2003) Phosphorylation-regulated cleavage of the tumor suppressor PTEN by caspase-3: implications for the control of protein stability and PTEN-protein interactions. J Biol Chem 278:30652–30660
Krippner-Heidenreich A, Talanian RV, Sekul R, Kraft R, Thole H, Ottleben H, Luscher B (2001) Targeting of the transcription factor Max during apoptosis: phosphorylation-regulated cleavage by caspase-5 at an unusual glutamic acid residue in position P1. Biochem J 358:705–715
Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC (2001) Protein kinase CK2 in mammary gland tumorigenesis. Oncogene 20:3247–3257
Munstermann U, Fritz G, Seitz G, Lu YP, Schneider HR, Issinger OG (1990) Casein kinase II is elevated in solid human tumours and rapidly proliferating non-neoplastic tissue. Eur J Biochem 189:251–257
Kim JS, Eom JI, Cheong JW, Choi AJ, Lee JK, Yang WI, Min YH (2007) Protein kinase CK2alpha as an unfavorable prognostic marker and novel therapeutic target in acute myeloid leukemia. Clin Cancer Res 13:1019–1028
Laramas M, Pasquier D, Filhol O, Ringeisen F, Descotes JL, Cochet C (2007) Nuclear localization of protein kinase CK2 catalytic subunit (CK2alpha) is associated with poor prognostic factors in human prostate cancer. Eur J Cancer 43:928–934
Wang G, Unger G, Ahmad KA, Slaton JW, Ahmed K (2005) Downregulation of CK2 induces apoptosis in cancer cells—a potential approach to cancer therapy. Mol Cell Biochem 274:77–84
Di Maira G, Brustolon F, Bertacchini J, Tosoni K, Marmiroli S, Pinna LA, Ruzzene M (2007) Pharmacological inhibition of protein kinase CK2 reverts the multidrug resistance phenotype of a CEM cell line characterized by high CK2 level. Oncogene 26:6915–6926
Siddiqui-Jain A, Drygin D, Streiner N, Chua P, Pierre F, O’Brien SE, Bliesath J, Omori M, Huser N, Ho C, Proffitt C, Schwaebe MK, Ryckman DM, Rice WG, Anderes K (2010) CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy. Cancer Res 70:10288–10298
Luthi AU, Martin SJ (2007) The CASBAH: a searchable database of caspase substrates. Cell Death Differ 14:641–650
Gnad F, Gunawardena J, Mann M (2011) PHOSIDA 2011: the posttranslational modification database. Nucleic Acids Res 39:D253–D260
Pradelli LA, Beneteau M, Ricci JE (2010) Mitochondrial control of caspase-dependent and -independent cell death. Cell Mol Life Sci 67:1589–1597
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME (1998) Two CD95 (APO-1/Fas) signaling pathways. EMBO J 17:1675–1687
Abdul-Ghani M, Megeney LA (2008) Rehabilitation of a contract killer: caspase-3 directs stem cell differentiation. Cell Stem Cell 2:515–516
Hashimoto T, Yamauchi L, Hunter T, Kikkawa U, Kamada S (2008) Possible involvement of caspase-7 in cell cycle progression at mitosis. Genes Cells 13:609–621
McComb S, Mulligan R, Sad S (2010) Caspase-3 is transiently activated without cell death during early antigen driven expansion of CD8(+) T cells in vivo. PLoS One 5:e15328
Demon D, Van Damme P, Vanden Berghe T, Deceuninck A, Van Durme J, Verspurten J, Helsens K, Impens F, Wejda M, Schymkowitz J, Rousseau F, Madder A, Vandekerckhove J, Declercq W, Gevaert K, Vandenabeele P (2009) Proteome-wide substrate analysis indicates substrate exclusion as a mechanism to generate caspase-7 versus caspase-3 specificity. Mol Cell Proteomics 8:2700–2714
McStay GP, Salvesen GS, Green DR (2008) Overlapping cleavage motif selectivity of caspases: implications for analysis of apoptotic pathways. Cell Death Differ 15:322–331
Hunter T (2000) Signaling–2000 and beyond. Cell 100:113–127
Hu Y, Yao J, Liu Z, Liu X, Fu H, Ye K (2005) Akt phosphorylates acinus and inhibits its proteolytic cleavage, preventing chromatin condensation. EMBO J 24:3543–3554
Bredemeyer AJ, Lewis RM, Malone JP, Davis AE, Gross J, Townsend RR, Ley TJ (2004) A proteomic approach for the discovery of protease substrates. Proc Natl Acad Sci USA 101:11785–11790
Hughes MA, Harper N, Butterworth M, Cain K, Cohen GM, MacFarlane M (2009) Reconstitution of the death-inducing signaling complex reveals a substrate switch that determines CD95-mediated death or survival. Mol Cell 35:265–279
Cursi S, Rufini A, Stagni V, Condo I, Matafora V, Bachi A, Bonifazi AP, Coppola L, Superti-Furga G, Testi R, Barila D (2006) Src kinase phosphorylates Caspase-8 on Tyr380: a novel mechanism of apoptosis suppression. EMBO J 25:1895–1905
Matthess Y, Raab M, Sanhaji M, Lavrik IN, Strebhardt K (2010) Cdk1/cyclin B1 controls Fas-mediated apoptosis by regulating caspase-8 activity. Mol Cell Biol 30(24):5726–5740
Su H, Bidere N, Zheng L, Cubre A, Sakai K, Dale J, Salmena L, Hakem R, Straus S, Lenardo M (2005) Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science 307:1465–1468
Seldin DC, Leder P (1995) Casein kinase II alpha transgene-induced murine lymphoma: relation to theileriosis in cattle. Science 267:894–897
Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Sonenshein GE (2002) Protein kinase CK2 promotes aberrant activation of nuclear factor-kappaB, transformed phenotype, and survival of breast cancer cells. Cancer Res 62:6770–6778
Ling P, Lu TJ, Yuan CJ, Lai MD (2008) Biosignaling of mammalian Ste20-related kinases. Cell Signal 20:1237–1247
Cheung WL, Ajiro K, Samejima K, Kloc M, Cheung P, Mizzen CA, Beeser A, Etkin LD, Chernoff J, Earnshaw WC, Allis CD (2003) Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell 113:507–517
Wen W, Zhu F, Zhang J, Keum YS, Zykova T, Yao K, Peng C, Zheng D, Cho YY, Ma WY, Bode AM, Dong Z (2010) MST1 promotes apoptosis through phosphorylation of histone H2AX. J Biol Chem 285(50):39108–39116
Qian Z, Lin C, Espinosa R, LeBeau M, Rosner MR (2001) Cloning and characterization of MST4, a novel Ste20-like kinase. J Biol Chem 276:22439–22445
Lin JL, Chen HC, Fang HI, Robinson D, Kung HJ, Shih HM (2001) MST4, a new Ste20-related kinase that mediates cell growth and transformation via modulating ERK pathway. Oncogene 20:6559–6569
Dan I, Ong SE, Watanabe NM, Blagoev B, Nielsen MM, Kajikawa E, Kristiansen TZ, Mann M, Pandey A (2002) Cloning of MASK, a novel member of the mammalian germinal center kinase III subfamily, with apoptosis-inducing properties. J Biol Chem 277:5929–5939
Lee KK, Murakawa M, Nishida E, Tsubuki S, Kawashima S, Sakamaki K, Yonehara S (1998) Proteolytic activation of MST/Krs, STE20-related protein kinase, by caspase during apoptosis. Oncogene 16:3029–3037
Graves JD, Gotoh Y, Draves KE, Ambrose D, Han DK, Wright M, Chernoff J, Clark EA, Krebs EG (1998) Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO J 17:2224–2234
Timmer JC, Zhu W, Pop C, Regan T, Snipas SJ, Eroshkin AM, Riedl SJ, Salvesen GS (2009) Structural and kinetic determinants of protease substrates. Nat Struct Mol Biol 16:1101–1108
Tadokoro D, Takahama S, Shimizu K, Hayashi S, Endo Y, Sawasaki T (2010) Characterization of a caspase-3-substrate kinome using an N- and C-terminally tagged protein kinase library produced by a cell-free system. Cell Death Dis 1:e89
Emoto Y, Manome Y, Meinhardt G, Kisaki H, Kharbanda S, Robertson M, Ghayur T, Wong WW, Kamen R, Weichselbaum R (1995) Proteolytic activation of protein kinase C delta by an ICE-like protease in apoptotic cells. EMBO J 14:6148–6156
Mizuno K, Noda K, Araki T, Imaoka T, Kobayashi Y, Akita Y, Shimonaka M, Kishi S, Ohno S (1997) The proteolytic cleavage of protein kinase C isotypes, which generates kinase and regulatory fragments, correlates with Fas-mediated and 12-O-tetradecanoyl-phorbol-13-acetate-induced apoptosis. Eur J Biochem 250:7–18
Haussermann S, Kittstein W, Rincke G, Johannes FJ, Marks F, Gschwendt M (1999) Proteolytic cleavage of protein kinase Cmu upon induction of apoptosis in U937 cells. FEBS Lett 462:442–446
Bordeaux MC, Forcet C, Granger L, Corset V, Bidaud C, Billaud M, Bredesen DE, Edery P, Mehlen P (2000) The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschsprung disease. EMBO J 19:4056–4063
Acknowledgments
This study has been funded by an operating grant from the Canadian Institute of Health Research (to DWL). JPT has been supported by scholarships from the Ontario Graduate Scholarship and Ontario Graduate Scholarship Science and Technology programs; JSD was supported by the Canadian Institute of Health Research—Canadian Graduate Scholarship and the Canadian Institute of Health Research—UWO Strategic Training Initiative in Cancer Research and Technology Transfer scholarship. The authors thank all the members of the Litchfield laboratory for holding helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turowec, J.P., Duncan, J.S., Gloor, G.B. et al. Regulation of caspase pathways by protein kinase CK2: identification of proteins with overlapping CK2 and caspase consensus motifs. Mol Cell Biochem 356, 159–167 (2011). https://doi.org/10.1007/s11010-011-0972-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0972-5