Abstract
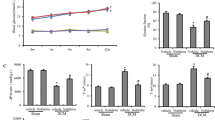
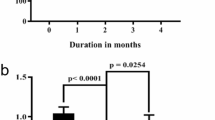
The present study was undertaken to evaluate the protective effects of genistein against cardiac inflammation and oxidative stress in streptozotocin (STZ) (45 mg/kg body weight)-induced diabetic rats. genistein (300 mg/kg/day) was administered orally for 24 weeks to STZ-induced diabetic rats. The effects of genistein on blood glucose, % glycosylated hemoglobin (HbA1c), C-reactive protein, tumor necrosis factor (TNF- α), transforming growth factor (TGF-β1), and total antioxidant were studied. Ultrastructural and histopathological assessment of injury were also undertaken using transmission electron microscope. STZ-induced diabetes resulted in significant increase in the levels of blood glucose, HbA1c, C-reactive protein, TNF- α and TGF-β1, and a decline in total antioxidant reserve of the myocardium. Administration of genistein to diabetic rats resulted in a decrease in blood glucose (p < 0.001), % HbA1c (p < 0.0001), C-reactive protein (p < 0.001), and expression of TNF- α (p < 0.001) and TGF-β1 (p < 0.0001) proteins. In addition, genistein treatment results in augmentation of total antioxidant (p < 0.01) reserve of the hearts. The above findings were supported by histological as well as immunohistochemical localization of NF-κB (p65) in the heart. Genistein treatment ameliorated the ultrastructural degenerative changes in the cardiac tissues as compared to the diabetic control. The result demonstrates that genistein restored the integrity of the diabetic myocardium by virtue of its anti-inflammatory and antioxidant effects.




Similar content being viewed by others
References
Dhalla NS, Liu X, Panagia V, Takeda N (1998) Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res 40:239–247
Mizushige K, Yao L, Noma T, Kiyomoto H, Yu Y, Hosomi N, Ohmori K, Matsuo H (2000) Alteration in left ventricular diastolic filling and accumulation of myocardial collagen at insulin-resistant prediabetic stage of a type II diabetic rat model. Circulation 101:899–907
Guan SJ, Ma ZH, Wu YL, Zhang JP, Liang F, Weiss JW, Guo QY, Wang JY, Ji ES, Chu L (2012) Long-term administration of fasudil improves cardiomyopathy in streptozotocin-induced diabetic rats. Food Chem Toxicol 50:1874–1882
Falcao-Pires I, Leite-Moreira AF (2012) Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail Rev 17:325–344
Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444:860–867
Stratmann B, Tschoepe D (2011) The diabetic heart: sweet, fatty and stressed. Expert Rev Cardiovasc Ther 9:1093–1096
Ku PM, Chen LJ, Liang JR, Cheng KC, Li YX, Cheng JT (2011) Molecular role of GATA binding protein 4 (GATA-4) in hyperglycemia-induced reduction of cardiac contractility. Cardiovasc Diabetol 10:1–15
Wen HL, Liang ZS, Zhang R, Yang K (2013) Anti-inflammatory effects of triptolide improve left ventricular function in a rat model of diabetic cardiomyopathy. Cardiovasc Diabetol 12:1–11
Wang G, Li W, Lu X, Bao P, Zhao X (2012) Luteolin ameliorates cardiac failure in type I diabetic cardiomyopathy. J Diabetes Complicat 4:259–265
Rösen P, Nawroth PP, King G, Möller W, Tritschler HJ, Packer L (2001) The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev 17:189–212
Westermann D, Van Linthout S, Dhayat S, Dhayat N, Schmidt A, Noutsias M, Song XY, Spillmann F, Riad A, Schultheiss HP, Tschöpe C (2007) Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol 6:500–507
Sara N, Edna S, Frederico P, Flávio R (2012) The role of inflammation in diabetic cardiomyopathy. Int J Infereron Cytokine Mediator Res 4:59–73
Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D (2002) Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 106:2067–2072
Patel S, Santani D (2009) Role of NF-kappa B in the pathogenesis of diabetes and its associated complications. Pharmacol Rep 4:595–603
Rutter MK, Meigs JB, Sullivan LM, D’Agostino RB Sr, Wilson PW (2004) C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation 110:380–385
Goyal BR, Mesariya P, Goyal RK, Mehta AA (2008) Effect of telmisartan on cardiovascular complications associated with streptozotocin diabetic rats. Mol Cell Biochem 314:123–131
Shah SJ, Marcus GM, Gerber IL, McKeown BH, Vessey JC, Jordan MV, Huddleston M, Foster E, Chatterjee K, Michaels AD (2006) High-sensitivity C-reactive protein and parameters of left ventricular dysfunction. J Card Fail 12:61–65
Kardys I, Knetsch AM, Bleumink GS, Deckers JW, Hofman A, Stricker BH, Witteman JC (2006) C-reactive protein and risk of heart failure: the Rotterdam Study. Am Heart J 152:514–520
Border WA, Noble NA (1995) Fibrosis linked to TGF-beta in yet another disease. J Clin Invest 2:655–656
Khan R, Sheppard R (2006) Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology 1:10–24
Hein S, Arnon E, Kostin S, Schonburg M, Elsasser A, Polyakova V, Bauer EP, Klovekorn WP, Schaper J (2003) Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation 7:984–991
Bugyei-Twum A, Advani A, Advani SL, Zhang Y, Thai K, Kelly DJ, Connelly KA (2014) High glucose induces Smad activation via the transcriptional coregulator p300 and contributes to cardiac fibrosis and hypertrophy. Cardiovasc Diabetol 13(89):1–12
Naderi GA, Asgary S, Sarraf-Zadegan N (2003) Anti-oxidant effect of flavonoids on the susceptibility of LDL oxidation. Mol Cell Biochem 246:193–196
Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N (1987) Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 12:5592–5595
Paradkar PN, Blum PS, Berhow MA, Baumann H, Kuo SM (2004) Dietary isoflavones suppress endotoxin-induced inflammatory reaction in liver and intestine. Cancer Lett 215:21–28
Park CE, Yun H, Lee EB, Min BI, Bae H, Choe W, Kang I, Kim SS, Ha J (2010) The antioxidant effects of genistein are associated with AMP-activated protein kinase activation and PTEN induction in prostate cancer cells. J Med Food 13:815–820
Gilbert ER, Liu D (2013) Anti-diabetic functions of soy isoflavone genistein: mechanisms underlying effects on pancreatic β-cell function. Food Funct 2:200–212
Nakajima M, Cooney MJ, Tu AH, Chang KY, Cao J, Ando A, An GJ, Melia M, de Juan E Jr (2001) Normalization of retinal vascular permeability in experimental diabetes with genistein. Invest Ophthalmol Vis Sci 42:2110–2114
Ramos JE, Al-Nakkash L, Peterson A, Gump BS, Janjulia T, Moore MS, Broderick TL, Carroll CC (2012) The soy isoflavone genistein inhibits the reduction in Achilles tendon collagen content induced by ovariectomy in rats. Scand J Med Sci Sports 5:e108–e114
Zeng X, Feng Y, Yang L, Huang Y, Zhou D, Sun J, Liu Y, Deng Y (2008) Single- and multiple-dose pharmacokinetics of genistein capsules in healthy chinese subjects: a phase I, randomized, open-label study. Curr Ther Res Clin Exp 4:318–333
Fouad AA, Al-Sultan AI, Yacoubi MT, Gomaa W (2010) Ameliorative effects of telmisartan in diabetic rats with indomethacin-induced gastric ulceration. Eur J Pharmacol 637:162–170
Yang W, Wang S, Li L, Liang Z, Wang L (2011) Genistein reduces hyperglycemia and islet cell loss in a high-dosage manner in rats with alloxan-induced pancreatic damage. Pancreas 40:396–402
Fu Z, Zhang W, Zhen W, Lum H, Nadler J, Bassaganya-Riera J, Jia Z, Wang Y, Misra H, Liu D (2010) Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology 151:3026–3037
Elmarakby AA, Ibrahim AS, Faulkner J, Mozaffari MS, Liou GI, Abdelsayed R (2011) Tyrosine kinase inhibitor, genistein, reduces renal inflammation and injury in streptozotocin-induced diabetic mice. Vasc Pharmacol 55:149–156
Lee JS (2006) Effects of soy protein and genistein on blood glucose, antioxidant enzyme activities, and lipid profile in streptozotocin-induced diabetic rats. Life Sci 79:1578–1584
Guo TL, Germolec DR, Zheng JF, Kooistra L, Auttachoat W, Smith MJ, White KL, Elmore SA (2014) Genistein protects female nonobese diabetic mice from developing type 1 diabetes when fed a soy- and alfalfa-free diet. Toxicol Pathol (Apr 8. [Epub ahead of print] PubMed PMID: 24713318)
Behloul N, Wu G (2013) Genistein: a promising therapeutic agent for obesity and diabetes treatment. Eur J Pharmacol 698:31–38
Nemoto O, Kawaguchi M, Yaoita H, Miyake K, Maehara K, Maruyama Y (2006) Left ventricular dysfunction and remodeling in streptozotocin-induced diabetic rats. Circ J 70:327–334
Van Linthout S, Spillmann F, Riad A, Trimpert C, Lievens J, Meloni M, Escher F, Filenberg E, Demir O, Li J, Shakibaei M, Schimke I, Staudt A, Felix SB, Schultheiss HP, De Geest B, Tschope C (2008) Human apolipoprotein A-I gene transfer reduces the development of experimental diabetic cardiomyopathy. Circulation 117:1563–1573
Sun D, Shen M, Li J, Li W, Zhang Y, Zhao L, Zhang Z, Yuan Y, Wang H, Cao F (2011) Cardioprotective effects of tanshinone IIA pretreatment via kinin B2 receptor-Akt-GSK-3b dependent pathway in experimental diabetic cardiomyopathy. Cardiovasc Diabetol 10:1–8
Rajesh M, Mukhopadhyay P, Batkai S, Patel V, Saito K, Matsumoto S, Kashiwaya Y, Horvath B, Mukhopadhyay B, Becker L, Hasko G, Liaudet L, Wink DA, Veves A, Mechoulam R, Pacher P (2010) Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol 56:2115–2125
Snell-Bergeon JK, West NA, Mayer-Davis EJ, Liese AD, Marcovina SM, D’Agostino RB Jr, Hamman RF, Dabelea D (2010) Inflammatory markers are increased in youth with type 1 diabetes: the SEARCH Case–control study. J Clin Endocrinol Metab 95:2868–2876
Nian M, Lee P, Khaper N, Liu P (2004) Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res 94:1543–1553
Ji G, Yang Q, Hao J, Guo L, Chen X, Hu J, Leng L, Jiang Z (2011) Anti-inflammatory effect of genistein on non-alcoholic steatohepatitis rats induced by high fat diet and its potential mechanisms. Int Immunopharmacol 6:762–768
Deodato B, Altavilla D, Squadrito G, Campo GM, Arlotta M, Minutoli L, Saitta A, Cucinotta D, Calapai G, Caputi AP, Miano M, Squadrito F (1999) Cardioprotection by the phytoestrogen genistein in experimental myocardial ischaemia-reperfusion injury. Br J Pharmacol 8:1683–1690
Hayashi H, Abdollah S, Qiu Y, Cai J, Xu YY, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA Jr, Wrana JL, Falb D (1997) The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGF beta signaling. Cell 7:1165–1173
Kim YS, Kim NH, Jung DH, Jang DS, Lee YM, Kim JM, Kim JS (2008) Genistein inhibits aldose reductase activity and high glucose-induced TGF-beta2 expression in human lens epithelial cells. Eur J Pharmacol 594:18–25
Kim H, Peterson TG, Barnes S (1998) Mechanisms of action of the soy isoflavone genistein: emerging role for its effects via transforming growth factor β signaling pathways1−3. Am J Clin Nutr 68:1418S–1425S
Agarwal A, Nick HS (2000) Renal response to tissue injury: lessons from heme oxygenase-1 gene ablation and expression. J Am Soc Nephrol 5:965–973
Ryter SW, Kim HP, Nakahira K, Zuckerbraun BS, Morse D, Choi AM (2007) Protective functions of heme oxygenase-1 and carbon monoxide in the respiratory system. Antioxid Redox Signal 9:2157–2173
Grieve DJ, Byme JA, Cave AC, Shah AM (2004) Role of oxidative stress in cardiac remodeling after myocardial infarction. Heart Lung Circ 13:132–138
Kameda K, Matsunaga T, Abe N, Hanada H, Ishizaka H, Ono H, Saitoh M, Fukui K, Fukuda I, Osanai T, Okumura K (2003) Correlation of oxidative stress with activity of matrix metalloproteinase in patients with coronary artery disease: possible role for left ventricular remodeling. Eur Heart J 24:2180–2185
Molavi B, Mehta JL (2004) Oxidative stress in cardiovascular disease: molecular basis of its deleterious effects, its detection, and therapeutic considerations. Curr Opin Cardiol 19:488–493
Boudina S, Abel ED (2007) Diabetic cardiomyopathy revisited. Circulation 115:3213–3223
Acknowledgments
Financial support provided by UKIERI and Department of Science and Technology (DST/INT/UK/P-39/2012), India is gratefully acknowledged. Facilities for electron microscopy availed at SAIF (DST), All India Institute of Medical Sciences, New Delhi, are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, S.K., Dongare, S., Mathur, R. et al. Genistein ameliorates cardiac inflammation and oxidative stress in streptozotocin-induced diabetic cardiomyopathy in rats. Mol Cell Biochem 408, 63–72 (2015). https://doi.org/10.1007/s11010-015-2483-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2483-2