Abstract
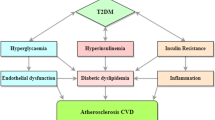
Coronary artery disease, the leading cause of death in the developed and developing countries, is prevalent in diabetes mellitus with 68% cardiovascular disease (CVD)-related mortality. Epidemiological studies suggested inverse correlation between HDL and CVD occurrence. Therefore, low HDL concentration observed in diabetic patients compared to non-diabetic individuals was thought to be one of the primary causes of increased risks of CVD. Efforts to raise HDL level via CETP inhibitors, Torcetrapib and Dalcetrapib, turned out to be disappointing in outcome studies despite substantial increases in HDL-C, suggesting that factors beyond HDL concentration may be responsible for the increased risks of CVD. Therefore, recent studies have focused more on HDL function than on HDL levels. The metabolic environment in diabetes mellitus condition such as hyperglycemia-induced advanced glycation end products, oxidative stress, and inflammation promote HDL dysfunction leading to greater risks of CVD. This review discusses dysfunctional HDL as one of the mechanisms of increased CVD risks in diabetes mellitus through adversely affecting components that support HDL function in cholesterol efflux and LDL oxidation. The dampening of reverse cholesterol transport, a key process that removes cholesterol from lipid-laden macrophages in the arterial wall, leads to increased risks of CVD in diabetic patients. Therapeutic approaches to keep diabetes under control may benefit patients from developing CVD.





Similar content being viewed by others
References
American Heart Association HDass-u (2007) A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 115:e69–e71
Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM (2004) Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 350:1495–1504. doi:10.1056/NEJMoa040583
Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801–809. doi:10.1038/362801a0
Ross R (1999) Atherosclerosis: an inflammatory disease. N Engl J Med 340:115–126. doi:10.1056/nejm199901143400207
Fonarow GC, Watson KE (2003) Effective strategies for long-term statin use. Am J Cardiol 92:27i–34i
Kastelein JJ (2003) The future of lipid-lowering therapy: the big picture. Neth J Med 61:35–39
Linsel-Nitschke P, Tall AR (2005) HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat Rev Drug Discov 4:193–205. doi:10.1038/nrd1658
Gordon DJ, Knoke J, Probstfield JL, Superko R, Tyroler HA (1986) High-density lipoprotein cholesterol and coronary heart disease in hypercholesterolemic men: the Lipid Research Clinics Coronary Primary Prevention Trial. Circulation 74:1217–1225
Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR Jr, Bangdiwala S, Tyroler HA (1989) High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 79:8–15
Hopkins PN, Heiss G, Ellison RC, Province MA, Pankow JS, Eckfeldt JH, Hunt SC (2003) Coronary artery disease risk in familial combined hyperlipidemia and familial hypertriglyceridemia: a case-control comparison from the National Heart, Lung, and Blood Institute Family Heart Study. Circulation 108:519–523. doi:10.1161/01.cir.0000081777.17879.85
Genest JJ, McNamara JR, Salem DN, Schaefer EJ (1991) Prevalence of risk factors in men with premature coronary artery disease. Am J Cardiol 67:1185–1189
Investigators DAIS (2001) Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet 357:905–910
Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, Manninen V et al (1987) Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 317:1237–1245. doi:10.1056/nejm198711123172001
Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J (1999) Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 341:410–418. doi:10.1056/nejm199908053410604
Reaven GM (1995) Pathophysiology of insulin resistance in human disease. Physiol Rev 75:473–486
Moller DE, Kaufman KD (2005) Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med 56:45–62. doi:10.1146/annurev.med.56.082103.104751
Srivastava RA, Srivastava N (2004) Search for obesity drugs: targeting central and peripheral pathways. Curr Med Chem 4:75–90
Hossain P, Kawar B, El Nahas M (2007) Obesity and diabetes in the developing world: a growing challenge. N Engl J Med 356:213–215. doi:10.1056/NEJMp068177
Srivastava RA, Srivastava N (2000) High density lipoprotein, apolipoprotein A-I, and coronary artery disease. Mol Cell Biochem 209:131–144
Choudhury RP, Rong JX, Trogan E, Elmalem VI, Dansky HM, Breslow JL, Witztum JL, Fallon JT, Fisher EA (2004) High-density lipoproteins retard the progression of atherosclerosis and favorably remodel lesions without suppressing indices of inflammation or oxidation. Arterioscler Thromb Vasc Biol 24:1904–1909. doi:10.1161/01.atv.0000142808.34602.25
Farbstein D, Levy AP (2012) HDL dysfunction in diabetes: causes and possible treatments. Expert Rev Cardiovasc Ther 10:353–361. doi:10.1586/erc.11.182
Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M (1996) Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 271:518–520
Van Eck M, Pennings M, Hoekstra M, Out R, Van Berkel TJ (2005) Scavenger receptor BI and ATP-binding cassette transporter A1 in reverse cholesterol transport and atherosclerosis. Curr Opin Lipidol 16:307–315
Joyce CW, Amar MJ, Lambert G, Vaisman BL, Paigen B, Najib-Fruchart J, Hoyt RF Jr, Neufeld ED, Remaley AT, Fredrickson DS, Brewer HB Jr, Santamarina-Fojo S (2002) The ATP binding cassette transporter A1 (ABCA1) modulates the development of aortic atherosclerosis in C57BL/6 and apoE-knockout mice. Proc Natl Acad Sci USA 99:407–412. doi:10.1073/pnas.012587699
Srivastava N (2002) ATP binding cassette transporter A1–key roles in cellular lipid transport and atherosclerosis. Mol Cell Biochem 237:155–164
Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA (2005) ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab 1:121–131. doi:10.1016/j.cmet.2005.01.002
Fournier N, de la Llera Moya M, Burkey BF, Swaney JB, Paterniti J Jr, Moatti N, Atger V, Rothblat GH (1996) Role of HDL phospholipid in efflux of cell cholesterol to whole serum: studies with human apoA-I transgenic rats. J Lipid Res 37:1704–1711
Bielicki JK, Johnson WJ, Weinberg RB, Glick JM, Rothblat GH (1992) Efflux of lipid from fibroblasts to apolipoproteins: dependence on elevated levels of cellular unesterified cholesterol. J Lipid Res 33:1699–1709
Smith JD, Miyata M, Ginsberg M, Grigaux C, Shmookler E, Plump AS (1996) Cyclic AMP induces apolipoprotein E binding activity and promotes cholesterol efflux from a macrophage cell line to apolipoprotein acceptors. J Biol Chem 271:30647–30655
Sakr SW, Williams DL, Stoudt GW, Phillips MC, Rothblat GH (1999) Induction of cellular cholesterol efflux to lipid-free apolipoprotein A-I by cAMP. Biochim Biophys Acta 1438:85–98
Li Q, Czarnecka H, Yokoyama S (1995) Involvement of a cellular surface factor(s) in lipid-free apolipoprotein-mediated cellular cholesterol efflux. Biochim Biophys Acta 1259:227–234
Mendez AJ, Oram JF (1997) Limited proteolysis of high density lipoprotein abolishes its interaction with cell-surface binding sites that promote cholesterol efflux. Biochim Biophys Acta 1346:285–299
Srivastava N, Chowdhury PR, Averna M, Srivastava RA (2001) Estrogen increases hepatic lipase levels in inbred strains of mice: a possible mechanism for estrogen-dependent lowering of high density lipoprotein. Mol Cell Biochem 220:87–93
Attie AD, Kastelein JP, Hayden MR (2001) Pivotal role of ABCA1 in reverse cholesterol transport influencing HDL levels and susceptibility to atherosclerosis. J Lipid Res 42:1717–1726
Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J Jr, Hayden MR (1999) Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet 22:336–345. doi:10.1038/11905
Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G (1999) The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet 22:347–351. doi:10.1038/11914
Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denefle P, Assmann G (1999) Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet 22:352–355. doi:10.1038/11921
Oram JF, Lawn RM, Garvin MR, Wade DP (2000) ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J Biol Chem 275:34508–34511. doi:10.1074/jbc.M006738200
Francone OL, Royer L, Haghpassand M (1996) Increased prebeta-HDL levels, cholesterol efflux, and LCAT-mediated esterification in mice expressing the human cholesteryl ester transfer protein (CETP) and human apolipoprotein A-I (apoA-I) transgenes. J Lipid Res 37:1268–1277
Wroblewska M (2011) The origin and metabolism of a nascent pre-beta high density lipoprotein involved in cellular cholesterol efflux. Acta Biochim Pol 58:275–285
Chroni A, Koukos G, Duka A, Zannis VI (2007) The carboxy-terminal region of apoA-I is required for the ABCA1-dependent formation of alpha-HDL but not prebeta-HDL particles in vivo. Biochemistry 46:5697–5708. doi:10.1021/bi602354t
Troutt JS, Alborn WE, Mosior MK, Dai J, Murphy AT, Beyer TP, Zhang Y, Cao G, Konrad RJ (2008) An apolipoprotein A-I mimetic dose-dependently increases the formation of prebeta1 HDL in human plasma. J Lipid Res 49:581–587. doi:10.1194/jlr.M700385-JLR200
Avdulov NA, Chochina SV, Igbavboa U, Wood WG (2000) Cholesterol efflux to high-density lipoproteins and apolipoprotein A-I phosphatidylcholine complexes is inhibited by ethanol: role of apolipoprotein structure and cooperative interaction of phosphatidylcholine and cholesterol. Biochemistry 39:10599–10606
Rye KA, Barter PJ (2004) Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler Thromb Vasc Biol 24:421–428. doi:10.1161/01.ATV.0000104029.74961.f5
Kane JP, Malloy MJ (2012) Prebeta-1 HDL and coronary heart disease. Curr Opin Lipidol 23:367–371. doi:10.1097/MOL.0b013e328353eef1
Vedhachalam C, Duong PT, Nickel M, Nguyen D, Dhanasekaran P, Saito H, Rothblat GH, Lund-Katz S, Phillips MC (2007) Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J Biol Chem 282:25123–25130. doi:10.1074/jbc.M704590200
Gelissen IC, Harris M, Rye KA, Quinn C, Brown AJ, Kockx M, Cartland S, Packianathan M, Kritharides L, Jessup W (2006) ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler Thromb Vasc Biol 26:534–540. doi:10.1161/01.ATV.0000200082.58536.e1
Dastani Z, Dangoisse C, Boucher B, Desbiens K, Krimbou L, Dufour R, Hegele RA, Pajukanta P, Engert JC, Genest J, Marcil M (2006) A novel nonsense apolipoprotein A-I mutation (apoA-I(E136X)) causes low HDL cholesterol in French Canadians. Atherosclerosis 185:127–136. doi:10.1016/j.atherosclerosis.2005.05.028
Koukos G, Chroni A, Duka A, Kardassis D, Zannis VI (2007) LCAT can rescue the abnormal phenotype produced by the natural ApoA-I mutations (Leu141Arg)Pisa and (Leu159Arg)FIN. Biochemistry 46:10713–10721. doi:10.1021/bi7003203
Savel J, Lafitte M, Pucheu Y, Pradeau V, Tabarin A, Couffinhal T (2012) Very low levels of HDL cholesterol and atherosclerosis, a variable relationship: a review of LCAT deficiency. Vasc Health Risk Manag 8:357–361. doi:10.2147/vhrm.s29985
Persegol L, Brindisi MC, Rageot D, Pais de Barros JP, Monier S, Verges B, Duvillard L (2015) Oxidation-induced loss of the ability of HDL to counteract the inhibitory effect of oxidized LDL on vasorelaxation. Heart Vessels 30:845–849. doi:10.1007/s00380-014-0543-2
Hine D, Mackness B, Mackness M (2011) Cholesteryl-ester transfer protein enhances the ability of high-density lipoprotein to inhibit low-density lipoprotein oxidation. IUBMB Life 63:772–774. doi:10.1002/iub.508
Hine D, Mackness B, Mackness M (2012) Coincubation of PON1, APO A1, and LCAT increases the time HDL is able to prevent LDL oxidation. IUBMB Life 64:157–161. doi:10.1002/iub.588
Huang Y, Wu Z, Riwanto M, Gao S, Levison BS, Gu X, Fu X, Wagner MA, Besler C, Gerstenecker G, Zhang R, Li XM, DiDonato AJ, Gogonea V, Tang WH, Smith JD, Plow EF, Fox PL, Shih DM, Lusis AJ, Fisher EA, DiDonato JA, Landmesser U, Hazen SL (2013) Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J Clin Invest 123:3815–3828. doi:10.1172/jci67478
Smith JD (2010) Myeloperoxidase, inflammation, and dysfunctional high-density lipoprotein. J Clin Lipidol 4:382–388. doi:10.1016/j.jacl.2010.08.007
Shao B, Oda MN, Oram JF, Heinecke JW (2010) Myeloperoxidase: an oxidative pathway for generating dysfunctional high-density lipoprotein. Chem Res Toxicol 23:447–454. doi:10.1021/tx9003775
Blatter Garin MC, Moren X, James RW (2006) Paraoxonase-1 and serum concentrations of HDL-cholesterol and apoA-I. J Lipid Res 47:515–520. doi:10.1194/jlr.M500281-JLR200
Garcia-Heredia A, Marsillach J, Rull A, Triguero I, Fort I, Mackness B, Mackness M, Shih DM, Joven J, Camps J (2013) Paraoxonase-1 inhibits oxidized low-density lipoprotein-induced metabolic alterations and apoptosis in endothelial cells: a nondirected metabolomic study. Mediators Inflamm 2013:156053. doi:10.1155/2013/156053
Rozenberg O, Shih DM, Aviram M (2005) Paraoxonase 1 (PON1) attenuates macrophage oxidative status: studies in PON1 transfected cells and in PON1 transgenic mice. Atherosclerosis 181:9–18. doi:10.1016/j.atherosclerosis.2004.12.030
Mackness B, Quarck R, Verreth W, Mackness M, Holvoet P (2006) Human paraoxonase-1 overexpression inhibits atherosclerosis in a mouse model of metabolic syndrome. Arterioscler Thromb Vasc Biol 26:1545–1550. doi:10.1161/01.ATV.0000222924.62641.aa
Tward A, Xia YR, Wang XP, Shi YS, Park C, Castellani LW, Lusis AJ, Shih DM (2002) Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation 106:484–490
Rozenberg O, Rosenblat M, Coleman R, Shih DM, Aviram M (2003) Paraoxonase (PON1) deficiency is associated with increased macrophage oxidative stress: studies in PON1-knockout mice. Free Radic Biol Med 34:774–784
Shao B, Oda MN, Vaisar T, Oram JF, Heinecke JW (2006) Pathways for oxidation of high-density lipoprotein in human cardiovascular disease. Curr Opin Mol Ther 8:198–205
Heinecke JW (2007) The role of myeloperoxidase in HDL oxidation and atherogenesis. Curr Atheroscler Rep 9:249–251
Shao B, Cavigiolio G, Brot N, Oda MN, Heinecke JW (2008) Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. Proc Natl Acad Sci USA 105:12224–12229. doi:10.1073/pnas.0802025105
Shao B, Tang C, Heinecke JW, Oram JF (2010) Oxidation of apolipoprotein A-I by myeloperoxidase impairs the initial interactions with ABCA1 required for signaling and cholesterol export. J Lipid Res 51:1849–1858. doi:10.1194/jlr.M004085
Kotani K, Sakane N, Ueda M, Mashiba S, Hayase Y, Tsuzaki K, Yamada T, Remaley AT (2012) Oxidized high-density lipoprotein is associated with increased plasma glucose in non-diabetic dyslipidemic subjects. Clin Chim Acta 414:125–129. doi:10.1016/j.cca.2012.08.021
Sampaio E, Barbosa DS, Mazzuco TL, Nunes VS, Passarelli M, Nakandakare ER, Carrilho AJ (2013) Impaired antioxidant action of high density lipoprotein in patients with type 1 diabetes with normoalbuminuria and microalbuminuria. Diabetes Res Clin Pract 99:321–326. doi:10.1016/j.diabres.2012.12.012
Kaysen GA (2009) Potential restoration of HDL function with apolipoprotein A-I mimetic peptide in end-stage renal disease. Kidney Int 76:359–361. doi:10.1038/ki.2009.205
Motamed M, Nargesi AA, Heidari B, Mirmiranpour H, Esteghamati A, Nakhjavani M (2016) Oxidized low-density lipoprotein (ox-LDL) to LDL ratio (ox-LDL/LDL) and ox-LDL to high-density lipoprotein ratio (ox-LDL/HDL). Clin Lab 62:1609–1617. doi:10.7754/Clin.Lab.2016.150412
Girona J, Manzanares JM, Marimon F, Cabre A, Heras M, Guardiola M, Ribalta J, Masana L (2008) Oxidized to non-oxidized lipoprotein ratios are associated with arteriosclerosis and the metabolic syndrome in diabetic patients. Nutr Metab Cardiovasc Dis 18:380–387. doi:10.1016/j.numecd.2007.04.002
Jurek A, Turyna B, Kubit P, Klein A (2006) LDL susceptibility to oxidation and HDL antioxidant capacity in patients with renal failure. Clin Biochem 39:19–27. doi:10.1016/j.clinbiochem.2005.08.009
Ng DS, Leiter LA, Vezina C, Connelly PW, Hegele RA (1994) Apolipoprotein A-I Q[-2]X causing isolated apolipoprotein A-I deficiency in a family with analphalipoproteinemia. J Clin Invest 93:223–229. doi:10.1172/jci116949
Miller M, Aiello D, Pritchard H, Friel G, Zeller K (1998) Apolipoprotein A-I(Zavalla) (Leu159→Pro): HDL cholesterol deficiency in a kindred associated with premature coronary artery disease. Arterioscler Thromb Vasc Biol 18:1242–1247
Santos RD, Schaefer EJ, Asztalos BF, Polisecki E, Wang J, Hegele RA, Martinez LR, Miname MH, Rochitte CE, Da Luz PL, Maranhao RC (2008) Characterization of high density lipoprotein particles in familial apolipoprotein A-I deficiency. J Lipid Res 49:349–357. doi:10.1194/jlr.M700362-JLR200
Gigante M, Ranieri E, Cerullo G, Calabresi L, Iolascon A, Assmann G, Morrone L, Pisciotta L, Schena FP, Gesualdo L (2006) LCAT deficiency: molecular and phenotypic characterization of an Italian family. J Nephrol 19:375–381
Holleboom AG, Kuivenhoven JA, Peelman F, Schimmel AW, Peter J, Defesche JC, Kastelein JJ, Hovingh GK, Stroes ES, Motazacker MM (2011) High prevalence of mutations in LCAT in patients with low HDL cholesterol levels in The Netherlands: identification and characterization of eight novel mutations. Hum Mutat 32:1290–1298. doi:10.1002/humu.21578
Kosmas CE, DeJesus E, Rosario D, Vittorio TJ (2016) CETP inhibition: past failures and future hopes. Clin Med Insights Cardiol 10:37–42. doi:10.4137/cmc.s32667
Chen Z, Wang SP, Krsmanovic ML, Castro-Perez J, Gagen K, Mendoza V, Rosa R, Shah V, He T, Stout SJ, Geoghagen NS, Lee SH, McLaren DG, Wang L, Roddy TP, Plump AS, Hubbard BK, Sinz CJ, Johns DG (2012) Small molecule activation of lecithin cholesterol acyltransferase modulates lipoprotein metabolism in mice and hamsters. Metabolism 61:470–481. doi:10.1016/j.metabol.2011.08.006
Freeman LA, Demosky SJ Jr, Konaklieva M, Kuskovsky R, Aponte A, Ossoli AF, Gordon SM, Koby RF, Manthei KA, Shen M, Vaisman BL, Shamburek RD, Jadhav A, Calabresi L, Gucek M, Tesmer JJG, Levine RL, Remaley AT (2017) Lecithin: cholesterol acyltransferase activation by sulfhydryl-reactive small molecules: role of cysteine-31. J Pharmacol Exp Ther 362:306–318. doi:10.1124/jpet.117.240457
Chenevard R, Hurlimann D, Spieker L, Bechir M, Enseleit F, Hermann M, Flammer AJ, Sudano I, Corti R, Luscher TF, Noll G, Ruschitzka F (2012) Reconstituted HDL in acute coronary syndromes. Cardiovasc Ther 30:e51–e57. doi:10.1111/j.1755-5922.2010.00221.x
Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B (2007) Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 357:2109–2122. doi:10.1056/NEJMoa0706628
Connelly MA, Parry TJ, Giardino EC, Huang Z, Cheung WM, Chen C, Cools F, Van der Linde H, Gallacher DJ, Kuo GH, Sarich TC, Demarest KT, Damiano BP (2010) Torcetrapib produces endothelial dysfunction independent of cholesteryl ester transfer protein inhibition. J Cardiovasc Pharmacol 55:459–468. doi:10.1097/FJC.0b013e3181cf03cb
Simic B, Hermann M, Shaw SG, Bigler L, Stalder U, Dorries C, Besler C, Luscher TF, Ruschitzka F (2012) Torcetrapib impairs endothelial function in hypertension. Eur Heart J 33:1615–1624. doi:10.1093/eurheartj/ehr348
Luscher TF, Taddei S, Kaski JC, Jukema JW, Kallend D, Munzel T, Kastelein JJ, Deanfield JE (2012) Vascular effects and safety of dalcetrapib in patients with or at risk of coronary heart disease: the dal-VESSEL randomized clinical trial. Eur Heart J 33:857–865. doi:10.1093/eurheartj/ehs019
Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, Rothblat GH (2007) The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res 48:2453–2462. doi:10.1194/jlr.M700274-JLR200
Annema W, Tietge UJ (2012) Regulation of reverse cholesterol transport: a comprehensive appraisal of available animal studies. Nutr Metab (Lond) 9:25. doi:10.1186/1743-7075-9-25
Whitlock ME, Swenson TL, Ramakrishnan R, Leonard MT, Marcel YL, Milne RW, Tall AR (1989) Monoclonal antibody inhibition of cholesteryl ester transfer protein activity in the rabbit. Effects on lipoprotein composition and high density lipoprotein cholesteryl ester metabolism. J Clin Invest 84:129–137. doi:10.1172/jci114132
Liu M, Chen Y, Zhang L, Wang Q, Ma X, Li X, Xiang R, Zhu Y, Qin S, Yu Y, Jiang XC, Duan Y, Han J (2015) Regulation of hepatic cholesteryl ester transfer protein expression and reverse cholesterol transport by inhibition of DNA topoisomerase II. J Biol Chem 290:14418–14429. doi:10.1074/jbc.M115.643015
Arai T, Wang N, Bezouevski M, Welch C, Tall AR (1999) Decreased atherosclerosis in heterozygous low density lipoprotein receptor-deficient mice expressing the scavenger receptor BI transgene. J Biol Chem 274:2366–2371
Webb NR, de Beer MC, Yu J, Kindy MS, Daugherty A, van der Westhuyzen DR, de Beer FC (2002) Overexpression of SR-BI by adenoviral vector promotes clearance of apoA-I, but not apoB, in human apoB transgenic mice. J Lipid Res 43:1421–1428
Trigatti B, Rayburn H, Vinals M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Rigotti A, Krieger M (1999) Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci USA 96:9322–9327
Van Eck M, Twisk J, Hoekstra M, Van Rij BT, Van der Lans CA, Bos IS, Kruijt JK, Kuipers F, Van Berkel TJ (2003) Differential effects of scavenger receptor BI deficiency on lipid metabolism in cells of the arterial wall and in the liver. J Biol Chem 278:23699–23705. doi:10.1074/jbc.M211233200
Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ (2007) Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest 117:2216–2224. doi:10.1172/jci32057
Zhang Y, Da Silva JR, Reilly M, Billheimer JT, Rothblat GH, Rader DJ (2005) Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest 115:2870–2874. doi:10.1172/jci25327
Van Eck M, Hoekstra M, Hildebrand RB, Yaong Y, Stengel D, Kruijt JK, Sattler W, Tietge UJ, Ninio E, Van Berkel TJ, Pratico D (2007) Increased oxidative stress in scavenger receptor BI knockout mice with dysfunctional HDL. Arterioscler Thromb Vasc Biol 27:2413–2419. doi:10.1161/atvbaha.107.145474
Zanoni P, Khetarpal SA, Larach DB, Hancock-Cerutti WF, Millar JS, Cuchel M, DerOhannessian S, Kontush A, Surendran P, Saleheen D, Trompet S, Jukema JW, De Craen A, Deloukas P, Sattar N, Ford I, Packard C, Majumder A, Alam DS, Di Angelantonio E, Abecasis G, Chowdhury R, Erdmann J, Nordestgaard BG, Nielsen SF, Tybjaerg-Hansen A, Schmidt RF, Kuulasmaa K, Liu DJ, Perola M, Blankenberg S, Salomaa V, Mannisto S, Amouyel P, Arveiler D, Ferrieres J, Muller-Nurasyid M, Ferrario M, Kee F, Willer CJ, Samani N, Schunkert H, Butterworth AS, Howson JM, Peloso GM, Stitziel NO, Danesh J, Kathiresan S, Rader DJ (2016) Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science 351:1166–1171. doi:10.1126/science.aad3517
Di Bartolo BA, Scherer DJ, Nicholls SJ (2016) Inducing apolipoprotein A-I synthesis to reduce cardiovascular risk: from ASSERT to SUSTAIN and beyond. Arch Med Sci 12:1302–1307. doi:10.5114/aoms.2016.62906
Balder JW, Staels B, Kuivenhoven JA (2013) Pharmacological interventions in human HDL metabolism. Curr Opin Lipidol 24:500–509. doi:10.1097/mol.0000000000000018
McLure KG, Gesner EM, Tsujikawa L, Kharencko OA, Attwell S, Campeau E, Wasiak S, Stein A, White A, Fontano E, Suto RK, Wong NC, Wagner GS, Hansen HC, Young PR (2013) RVX-208, An Inducer of apoA-I in Humans, is a BET Bromodomain Antagonist. PLoS ONE 8:e83190
Siebel AL, Trinh SK, Formosa MF, Mundra PA, Natoli AK, Reddy-Luthmoodoo M, Huynh K, Khan AA, Carey AL, van Hall G, Cobelli C, Dalla-Man C, Otvos JD, Rye KA, Johansson J, Gordon A, Wong NC, Sviridov D, Barter P, Duffy SJ, Meikle PJ, Kingwell BA (2016) Effects of the BET-inhibitor, RVX-208 on the HDL lipidome and glucose metabolism in individuals with prediabetes: a randomized controlled trial. Metabolism 65:904–914. doi:10.1016/j.metabol.2016.03.002
Kleinman JC, Donahue RP, Harris MI, Finucane FF, Madans JH, Brock DB (1988) Mortality among diabetics in a national sample. Am J Epidemiol 128:389–401
Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF (2010) Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 8:29. doi:10.1186/1478-7954-8-29
Ford ES, Giles WH, Dietz WH (2002) Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 287:356–359
Gatti A, Maranghi M, Bacci S, Carallo C, Gnasso A, Mandosi E, Fallarino M, Morano S, Trischitta V, Filetti S (2009) Poor glycemic control is an independent risk factor for low HDL cholesterol in patients with type 2 diabetes. Diabetes Care 32:1550–1552. doi:10.2337/dc09-0256
Abou-Seif MA, Youssef AA (2004) Evaluation of some biochemical changes in diabetic patients. Clin Chim Acta 346:161–170. doi:10.1016/j.cccn.2004.03.030
Ohgami N, Miyazaki A, Sakai M, Kuniyasu A, Nakayama H, Horiuchi S (2003) Advanced glycation end products (AGE) inhibit scavenger receptor class B type I-mediated reverse cholesterol transport: a new crossroad of AGE to cholesterol metabolism. J Atheroscler Thromb 10:1–6
Hoang A, Murphy AJ, Coughlan MT, Thomas MC, Forbes JM, O’Brien R, Cooper ME, Chin-Dusting JP, Sviridov D (2007) Advanced glycation of apolipoprotein A-I impairs its anti-atherogenic properties. Diabetologia 50:1770–1779. doi:10.1007/s00125-007-0718-9
Drew BG, Duffy SJ, Formosa MF, Natoli AK, Henstridge DC, Penfold SA, Thomas WG, Mukhamedova N, de Courten B, Forbes JM, Yap FY, Kaye DM, van Hall G, Febbraio MA, Kemp BE, Sviridov D, Steinberg GR, Kingwell BA (2009) High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation 119:2103–2111. doi:10.1161/circulationaha.108.843219
Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP (1992) Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes 41:715–722
Waldman B, Jenkins AJ, Davis TM, Taskinen MR, Scott R, O’Connell RL, Gebski VJ, Ng MK, Keech AC (2014) HDL-C and HDL-C/ApoA-I predict long-term progression of glycemia in established type 2 diabetes. Diabetes Care 37:2351–2358. doi:10.2337/dc13-2738
Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA (2002) Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med 347:1483–1492. doi:10.1056/NEJMoa020194
Quintao EC, Medina WL, Passarelli M (2000) Reverse cholesterol transport in diabetes mellitus. Diabetes Metab Res Rev 16:237–250
Cavallero E, Brites F, Delfly B, Nicolaiew N, Decossin C, De Geitere C, Fruchart JC, Wikinski R, Jacotot B, Castro G (1995) Abnormal reverse cholesterol transport in controlled type II diabetic patients. Studies on fasting and postprandial LpA-I particles. Arterioscler Thromb Vasc Biol 15:2130–2135
Capaldo B, Di Bonito P, Iaccarino M, Roman MJ, Lee ET, Devereux RB, Riccardi G, Howard BV, de Simone G (2012) Cardiovascular characteristics in subjects with increasing levels of abnormal glucose regulation: the Strong Heart Study. Diabetes Care. doi:10.2337/dc12-1501
Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW (2014) HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 371:2383–2393. doi:10.1056/NEJMoa1409065
Rohatgi A, Grundy SM (2015) Cholesterol efflux capacity as a therapeutic target: rationale and clinical implications. J Am Coll Cardiol 66:2211–2213. doi:10.1016/j.jacc.2015.09.012
Kubota M, Nakanishi S, Hirano M, Maeda S, Yoneda M, Awaya T, Yamane K, Kohno N (2014) Relationship between serum cholesterol efflux capacity and glucose intolerance in Japanese-Americans. J Atheroscler Thromb 21:1087–1097
Passarelli M, Tang C, McDonald TO, O’Brien KD, Gerrity RG, Heinecke JW, Oram JF (2005) Advanced glycation end product precursors impair ABCA1-dependent cholesterol removal from cells. Diabetes 54:2198–2205
Walcher D, Marx N (2009) Advanced glycation end products and C-peptide-modulators in diabetic vasculopathy and atherogenesis. Semin Immunopathol 31:103–111. doi:10.1007/s00281-009-0144-9
Pu LJ, Lu L, Zhang RY, Du R, Shen Y, Zhang Q, Yang ZK, Chen QJ, Shen WF (2012) Glycation of apoprotein A-I is associated with coronary artery plaque progression in type 2 diabetic patients. Diabetes Care. doi:10.2337/dc12-1411
Traldi P, Castilho G, Sartori CH, Machado-Lima A, Nakandakare ER, Correa-Giannella ML, Roverso M, Porcu S, Lapolla A, Passarelli M (2015) Glycated human serum albumin isolated from poorly controlled diabetic patients impairs cholesterol efflux from macrophages: an investigation by mass spectrometry. Eur J Mass Spectrom (Chichester, Eng) 21:233–244. doi:10.1255/ejms.1322
Machado-Lima A, Iborra RT, Pinto RS, Castilho G, Sartori CH, Oliveira ER, Okuda LS, Nakandakare ER, Giannella-Neto D, Machado UF, Correa-Giannella ML, Traldi P, Porcu S, Roverso M, Lapolla A, Passarelli M (2015) In type 2 diabetes mellitus glycated albumin alters macrophage gene expression impairing ABCA1-mediated cholesterol efflux. J Cell Physiol 230:1250–1257. doi:10.1002/jcp.24860
Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, Kastelein JJ, Boekholdt SM, Khaw KT, Wareham N, Rader DJ (2015) Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol 3:507–513. doi:10.1016/s2213-8587(15)00126-6
Bao LD, Li CQ, Peng R, Ren XH, Ma RL, Wang Y, Lv HJ (2015) Correlation between the decrease of cholesterol efflux from macrophages in patients with type II diabetes mellitus and down-regulated CYP7A1 expression. Genet Mol Res 14:8716–8724. doi:10.4238/2015.July.31.20
Apro J, Tietge UJ, Dikkers A, Parini P, Angelin B, Rudling M (2016) Impaired cholesterol efflux capacity of high-density lipoprotein isolated from interstitial fluid in type 2 diabetes mellitus-brief report. Arterioscler Thromb Vasc Biol 36:787–791. doi:10.1161/atvbaha.116.307385
Tsun JG, Yung S, Chau MK, Shiu SW, Chan TM, Tan KC (2014) Cellular cholesterol transport proteins in diabetic nephropathy. PLoS ONE 9:e105787. doi:10.1371/journal.pone.0105787
Manjunatha S, Distelmaier K, Dasari S, Carter RE, Kudva YC, Nair KS (2016) Functional and proteomic alterations of plasma high density lipoproteins in type 1 diabetes mellitus. Metabolism 65:1421–1431. doi:10.1016/j.metabol.2016.06.008
Attia N, Nakbi A, Smaoui M, Chaaba R, Moulin P, Hammami S, Hamda KB, Chanussot F, Hammami M (2007) Increased phospholipid transfer protein activity associated with the impaired cellular cholesterol efflux in type 2 diabetic subjects with coronary artery disease. Tohoku J Exp Med 213:129–137
Jaleel A, Henderson GC, Madden BJ, Klaus KA, Morse DM, Gopala S, Nair KS (2010) Identification of de novo synthesized and relatively older proteins: accelerated oxidative damage to de novo synthesized apolipoprotein A-1 in type 1 diabetes. Diabetes 59:2366–2374. doi:10.2337/db10-0371
Nobecourt E, Tabet F, Lambert G, Puranik R, Bao S, Yan L, Davies MJ, Brown BE, Jenkins AJ, Dusting GJ, Bonnet DJ, Curtiss LK, Barter PJ, Rye KA (2010) Nonenzymatic glycation impairs the antiinflammatory properties of apolipoprotein A-I. Arterioscler Thromb Vasc Biol 30:766–772. doi:10.1161/atvbaha.109.201715
Okuda LS, Castilho G, Rocco DD, Nakandakare ER, Catanozi S, Passarelli M (2012) Advanced glycated albumin impairs HDL anti-inflammatory activity and primes macrophages for inflammatory response that reduces reverse cholesterol transport. Biochim Biophys Acta 1821:1485–1492. doi:10.1016/j.bbalip.2012.08.011
Pajkrt D, Doran JE, Koster F, Lerch PG, Arnet B, van der Poll T, ten Cate JW, van Deventer SJ (1996) Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J Exp Med 184:1601–1608
Bursill CA, Castro ML, Beattie DT, Nakhla S, van der Vorst E, Heather AK, Barter PJ, Rye KA (2010) High-density lipoproteins suppress chemokines and chemokine receptors in vitro and in vivo. Arterioscler Thromb Vasc Biol 30:1773–1778. doi:10.1161/atvbaha.110.211342
Patel S, Drew BG, Nakhla S, Duffy SJ, Murphy AJ, Barter PJ, Rye KA, Chin-Dusting J, Hoang A, Sviridov D, Celermajer DS, Kingwell BA (2009) Reconstituted high-density lipoprotein increases plasma high-density lipoprotein anti-inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes. J Am Coll Cardiol 53:962–971. doi:10.1016/j.jacc.2008.12.008
Patel DC, Albrecht C, Pavitt D, Paul V, Pourreyron C, Newman SP, Godsland IF, Valabhji J, Johnston DG (2011) Type 2 diabetes is associated with reduced ATP-binding cassette transporter A1 gene expression, protein and function. PLoS ONE 6:e22142. doi:10.1371/journal.pone.0022142
Shao B, Pennathur S, Pagani I, Oda MN, Witztum JL, Oram JF, Heinecke JW (2010) Modifying apolipoprotein A-I by malondialdehyde, but not by an array of other reactive carbonyls, blocks cholesterol efflux by the ABCA1 pathway. J Biol Chem 285:18473–18484. doi:10.1074/jbc.M110.118182
Morgantini C, Natali A, Boldrini B, Imaizumi S, Navab M, Fogelman AM, Ferrannini E, Reddy ST (2011) Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes 60:2617–2623. doi:10.2337/db11-0378
McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP (2009) Inflammation impairs reverse cholesterol transport in vivo. Circulation 119:1135–1145. doi:10.1161/circulationaha.108.810721
Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL (2004) Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest 114:529–541. doi:10.1172/jci21109
Jornayvaz FR, Brulhart-Meynet MC, James RW (2009) Myeloperoxidase and paraoxonase-1 in type 2 diabetic patients. Nutr Metab Cardiovasc Dis 19:613–619. doi:10.1016/j.numecd.2008.12.005
Shao B, Pennathur S, Heinecke JW (2012) Myeloperoxidase targets apolipoprotein A-I, the major high density lipoprotein protein, for site-specific oxidation in human atherosclerotic lesions. J Biol Chem 287:6375–6386. doi:10.1074/jbc.M111.337345
Kappelle PJ, Bijzet J, Hazenberg BP, Dullaart RP (2011) Lower serum paraoxonase-1 activity is related to higher serum amyloid a levels in metabolic syndrome. Arch Med Res 42:219–225. doi:10.1016/j.arcmed.2011.05.002
Murakami H, Tanabe J, Tamasawa N, Matsumura K, Yamashita M, Matsuki K, Murakami H, Matsui J, Suda T (2013) Reduction of paraoxonase-1 activity may contribute the qualitative impairment of HDL particles in patients with type 2 diabetes. Diabetes Res Clin Pract 99:30–38. doi:10.1016/j.diabres.2012.10.022
Annema W, Nijstad N, Tolle M, de Boer JF, Buijs RV, Heeringa P, van der Giet M, Tietge UJ (2010) Myeloperoxidase and serum amyloid A contribute to impaired in vivo reverse cholesterol transport during the acute phase response but not group IIA secretory phospholipase A(2). J Lipid Res 51:743–754. doi:10.1194/jlr.M000323
Shao B, Oda MN, Oram JF, Heinecke JW (2006) Myeloperoxidase: an inflammatory enzyme for generating dysfunctional high density lipoprotein. Curr Opin Cardiol 21:322–328. doi:10.1097/01.hco.0000231402.87232.aa
Nicholls SJ, Zheng L, Hazen SL (2005) Formation of dysfunctional high-density lipoprotein by myeloperoxidase. Trends Cardiovasc Med 15:212–219. doi:10.1016/j.tcm.2005.06.004
Undurti A, Huang Y, Lupica JA, Smith JD, DiDonato JA, Hazen SL (2009) Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J Biol Chem 284:30825–30835. doi:10.1074/jbc.M109.047605
Peng DQ, Brubaker G, Wu Z, Zheng L, Willard B, Kinter M, Hazen SL, Smith JD (2008) Apolipoprotein A-I tryptophan substitution leads to resistance to myeloperoxidase-mediated loss of function. Arterioscler Thromb Vasc Biol 28:2063–2070. doi:10.1161/atvbaha.108.173815
Du X, Matsumura T, Edelstein D, Rossetti L, Zsengeller Z, Szabo C, Brownlee M (2003) Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest 112:1049–1057. doi:10.1172/jci18127
Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404:787–790. doi:10.1038/35008121
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820. doi:10.1038/414813a
Cacicedo JM, Benjachareowong S, Chou E, Ruderman NB, Ido Y (2005) Palmitate-induced apoptosis in cultured bovine retinal pericytes: roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes 54:1838–1845
Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H (2000) High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49:1939–1945
Ostrander DB, Sparagna GC, Amoscato AA, McMillin JB, Dowhan W (2001) Decreased cardiolipin synthesis corresponds with cytochrome c release in palmitate-induced cardiomyocyte apoptosis. J Biol Chem 276:38061–38067. doi:10.1074/jbc.M107067200
Ceriello A, Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A, Esposito K, Giugliano D (2004) Effect of postprandial hypertriglyceridemia and hyperglycemia on circulating adhesion molecules and oxidative stress generation and the possible role of simvastatin treatment. Diabetes 53:701–710
Huang Y, Didonato JA, Levison BS, Schmitt D, Li L, Wu Y, Buffa J, Kim T, Gerstenecker GS, Gu X, Kadiyala CS, Wang Z, Culley MK, Hazen JE, Didonato AJ, Fu X, Berisha SZ, Peng D, Nguyen TT, Liang S, Chuang CC, Cho L, Plow EF, Fox PL, Gogonea V, Tang WH, Parks JS, Fisher EA, Smith JD, Hazen SL (2014) An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat Med 20:193–203. doi:10.1038/nm.3459
Kataoka Y, Shao M, Wolski K, Uno K, Puri R, Murat Tuzcu E, Hazen SL, Nissen SE, Nicholls SJ (2014) Myeloperoxidase levels predict accelerated progression of coronary atherosclerosis in diabetic patients: insights from intravascular ultrasound. Atherosclerosis 232:377–383. doi:10.1016/j.atherosclerosis.2013.11.075
Shao B, Tang C, Sinha A, Mayer PS, Davenport GD, Brot N, Oda MN, Zhao XQ, Heinecke JW (2014) Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ Res 114:1733–1742. doi:10.1161/circresaha.114.303454
Lu N, Xie S, Li J, Tian R, Peng YY (2015) Myeloperoxidase-mediated oxidation targets serum apolipoprotein A-I in diabetic patients and represents a potential mechanism leading to impaired anti-apoptotic activity of high density lipoprotein. Clin Chim Acta 441:163–170. doi:10.1016/j.cca.2014.12.014
Chen X, Bakillah A, Zhou L, Pan X, Hoepfner F, Jacob M, Jiang XC, Lazar J, Schlitt A, Hussain MM (2016) Nitrated apolipoprotein AI/apolipoprotein AI ratio is increased in diabetic patients with coronary artery disease. Atherosclerosis 245:12–21. doi:10.1016/j.atherosclerosis.2015.11.021
Abderrahmani A, Niederhauser G, Favre D, Abdelli S, Ferdaoussi M, Yang JY, Regazzi R, Widmann C, Waeber G (2007) Human high-density lipoprotein particles prevent activation of the JNK pathway induced by human oxidised low-density lipoprotein particles in pancreatic beta cells. Diabetologia 50:1304–1314. doi:10.1007/s00125-007-0642-z
Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasanji Z, Marsh BJ, Rodrigues B, Johnson JD, Parks JS, Verchere CB, Hayden MR (2007) Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med 13:340–347. doi:10.1038/nm1546
Han R, Lai R, Ding Q, Wang Z, Luo X, Zhang Y, Cui G, He J, Liu W, Chen Y (2007) Apolipoprotein A-I stimulates AMP-activated protein kinase and improves glucose metabolism. Diabetologia 50:1960–1968. doi:10.1007/s00125-007-0752-7
Drew BG, Fidge NH, Gallon-Beaumier G, Kemp BE, Kingwell BA (2004) High-density lipoprotein and apolipoprotein AI increase endothelial NO synthase activity by protein association and multisite phosphorylation. Proc Natl Acad Sci USA 101:6999–7004. doi:10.1073/pnas.0306266101
Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R (2003) Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 290:2292–2300. doi:10.1001/jama.290.17.2292
Tardif JC, Gregoire J, L’Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodes-Cabau J (2007) Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA 297:1675–1682. doi:10.1001/jama.297.15.jpc70004
Nieuwdorp M, Vergeer M, Bisoendial RJ, Roodt J, Levels H, Birjmohun RS, Kuivenhoven JA, Basser R, Rabelink TJ, Kastelein JJ, Stroes ES (2008) Reconstituted HDL infusion restores endothelial function in patients with type 2 diabetes mellitus. Diabetologia 51:1081–1084. doi:10.1007/s00125-008-0975-2
Wang Y, Oram JF (2002) Unsaturated fatty acids inhibit cholesterol efflux from macrophages by increasing degradation of ATP-binding cassette transporter A1. J Biol Chem 277:5692–5697. doi:10.1074/jbc.M109977200
Reaven GM, Chen YD (1988) Role of abnormal free fatty acid metabolism in the development of non-insulin-dependent diabetes mellitus. Am J Med 85:106–112
Srivastava RA, Srivastava N, Averna M, Cefalu AB, Schonfeld G (1999) Molecular bases of low production rates of apolipoprotein B-100 and truncated apoB-82 in a mutant HepG2 cell line generated by targeted modification of the apolipoprotein B gene. J Lipid Res 40:901–912
Adeli K, Wettesten M, Asp L, Mohammadi A, Macri J, Olofsson SO (1997) Intracellular assembly and degradation of apolipoprotein B-100-containing lipoproteins in digitonin-permeabilized HEP G2 cells. J Biol Chem 272:5031–5039
Srivastava N, Cefalu AB, Noto D, Schonfeld G, Averna M, Srivastava RA (2010) The production of 85 kDa N-terminal fragment of apolipoprotein B in mutant HepG2 cells generated by targeted modification of apoB gene occurs by ALLN-inhibitable protease cleavage during translocation. Biochem Biophys Res Commun 398:665–670. doi:10.1016/j.bbrc.2010.06.130
Wu KK, Huan Y (2007) Diabetic atherosclerosis mouse models. Atherosclerosis 191:241–249. doi:10.1016/j.atherosclerosis.2006.08.030
Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ (2011) Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 364:127–135. doi:10.1056/NEJMoa1001689
Yamamoto S, Narita I, Kotani K (2016) The macrophage and its related cholesterol efflux as a HDL function index in atherosclerosis. Clin Chim Acta 457:117–122. doi:10.1016/j.cca.2016.04.012
Aiello RJ, Brees D, Bourassa PA, Royer L, Lindsey S, Coskran T, Haghpassand M, Francone OL (2002) Increased atherosclerosis in hyperlipidemic mice with inactivation of ABCA1 in macrophages. Arterioscler Thromb Vasc Biol 22:630–637
Ou X, Dai X, Long Z, Tang Y, Cao D, Hao X, Hu Y, Li X, Tang C (2008) Liver X receptor agonist T0901317 reduces atherosclerotic lesions in apoE−/− mice by up-regulating NPC1 expression. Sci. China C 51:418–429. doi:10.1007/s11427-008-0054-4
Brunham LR, Singaraja RR, Duong M, Timmins JM, Fievet C, Bissada N, Kang MH, Samra A, Fruchart JC, McManus B, Staels B, Parks JS, Hayden MR (2009) Tissue-specific roles of ABCA1 influence susceptibility to atherosclerosis. Arterioscler Thromb Vasc Biol 29:548–554. doi:10.1161/atvbaha.108.182303
Wang Y, Oram JF (2005) Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a phospholipase D2 pathway. J Biol Chem 280:35896–35903. doi:10.1074/jbc.M506210200
Wang Y, Oram JF (2007) Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a protein kinase C delta pathway. J Lipid Res 48:1062–1068. doi:10.1194/jlr.M600437-JLR200
Hawley SA, Gadalla AE, Olsen GS, Hardie DG (2002) The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 51:2420–2425
Hardie DG (2008) AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond) 32(Suppl 4):S7–S12. doi:10.1038/ijo.2008.116
Srivastava RA, Pinkosky SL, Filippov S, Hanselman JC, Cramer CT, Newton RS (2012) AMP-activated protein kinase: an emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J Lipid Res 53:2490–2514. doi:10.1194/jlr.R025882
Park KG, Min AK, Koh EH, Kim HS, Kim MO, Park HS, Kim YD, Yoon TS, Jang BK, Hwang JS, Kim JB, Choi HS, Park JY, Lee IK, Lee KU (2008) Alpha-lipoic acid decreases hepatic lipogenesis through adenosine monophosphate-activated protein kinase (AMPK)-dependent and AMPK-independent pathways. Hepatology 48:1477–1486. doi:10.1002/hep.22496
Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8:1288–1295. doi:10.1038/nm788
Lehti M, Donelan E, Abplanalp W, Al-Massadi O, Habegger KM, Weber J, Ress C, Mansfeld J, Somvanshi S, Trivedi C, Keuper M, Ograjsek T, Striese C, Cucuruz S, Pfluger PT, Krishna R, Gordon SM, Silva RA, Luquet S, Castel J, Martinez S, D’Alessio D, Davidson WS, Hofmann SM (2013) High-density lipoprotein maintains skeletal muscle function by modulating cellular respiration in mice. Circulation 128:2364–2371. doi:10.1161/circulationaha.113.001551
Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D (2003) LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol 13:2004–2008
Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG (2005) Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2:9–19. doi:10.1016/j.cmet.2005.05.009
Vaughan AM, Oram JF (2006) ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J Lipid Res 47:2433–2443. doi:10.1194/jlr.M600218-JLR200
Son SH, Goo YH, Choi M, Saha PK, Oka K, Chan LC, Paul A (2016) Enhanced atheroprotection and lesion remodelling by targeting the foam cell and increasing plasma cholesterol acceptors. Cardiovasc Res 109:294–304. doi:10.1093/cvr/cvv241
Lorenzi I, von Eckardstein A, Radosavljevic S, Rohrer L (2008) Lipidation of apolipoprotein A-I by ATP-binding cassette transporter (ABC) A1 generates an interaction partner for ABCG1 but not for scavenger receptor BI. Biochem Biophys Acta 1781:306–313. doi:10.1016/j.bbalip.2008.04.006
Yin K, Liao DF, Tang CK (2010) ATP-binding membrane cassette transporter A1 (ABCA1): a possible link between inflammation and reverse cholesterol transport. Mol Med 16:438–449. doi:10.2119/molmed.2010.00004
Yvan-Charvet L, Wang N, Tall AR (2010) Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol 30:139–143. doi:10.1161/atvbaha.108.179283
Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hui EK, Nayak DP, Fogelman AM (2004) D-4F, an apolipoprotein A-I mimetic peptide, inhibits the inflammatory response induced by influenza A infection of human type II pneumocytes. Circulation 110:3252–3258. doi:10.1161/01.CIR.0000147232.75456.B3
Barter PJ, Puranik R, Rye KA (2007) New insights into the role of HDL as an anti-inflammatory agent in the prevention of cardiovascular disease. Curr Cardiol Rep 9:493–498
Murphy AJ, Woollard KJ, Hoang A, Mukhamedova N, Stirzaker RA, McCormick SP, Remaley AT, Sviridov D, Chin-Dusting J (2008) High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol 28:2071–2077. doi:10.1161/atvbaha.108.168690
Tabet F, Remaley AT, Segaliny AI, Millet J, Yan L, Nakhla S, Barter PJ, Rye KA, Lambert G (2010) The 5A apolipoprotein A-I mimetic peptide displays antiinflammatory and antioxidant properties in vivo and in vitro. Arterioscler Thromb Vasc Biol 30:246–252. doi:10.1161/atvbaha.109.200196
de la Llera Moya M, McGillicuddy FC, Hinkle CC, Byrne M, Joshi MR, Nguyen V, Tabita-Martinez J, Wolfe ML, Badellino K, Pruscino L, Mehta NN, Asztalos BF, Reilly MP (2012) Inflammation modulates human HDL composition and function in vivo. Atherosclerosis. doi:10.1016/j.atherosclerosis.2012.02.032
Majdalawieh A, Ro HS (2009) LPS-induced suppression of macrophage cholesterol efflux is mediated by adipocyte enhancer-binding protein 1. Int J Biochem Cell Biol 41:1518–1525. doi:10.1016/j.biocel.2009.01.003
Yvan-Charvet L, Kling J, Pagler T, Li H, Hubbard B, Fisher T, Sparrow CP, Taggart AK, Tall AR (2010) Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler Thromb Vasc Biol 30:1430–1438. doi:10.1161/atvbaha.110.207142
Moore RE, Navab M, Millar JS, Zimetti F, Hama S, Rothblat GH, Rader DJ (2005) Increased atherosclerosis in mice lacking apolipoprotein A-I attributable to both impaired reverse cholesterol transport and increased inflammation. Circ Res 97:763–771. doi:10.1161/01.RES.0000185320.82962.F7
Yvan-Charvet L, Pagler TA, Seimon TA, Thorp E, Welch CL, Witztum JL, Tabas I, Tall AR (2010) ABCA1 and ABCG1 protect against oxidative stress-induced macrophage apoptosis during efferocytosis. Circ Res 106:1861–1869. doi:10.1161/circresaha.110.217281
Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Krankel N, Kania G, Zewinger S, Akhmedov A, Shi Y, Martin T, Perisa D, Winnik S, Muller MF, Sester U, Wernicke G, Jung A, Gutteck U, Eriksson U, Geisel J, Deanfield J, von Eckardstein A, Luscher TF, Fliser D, Bahlmann FH, Landmesser U (2013) Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 38:754–768. doi:10.1016/j.immuni.2013.02.009
Flegel WA, Baumstark MW, Weinstock C, Berg A, Northoff H (1993) Prevention of endotoxin-induced monokine release by human low- and high-density lipoproteins and by apolipoprotein A-I. Infect Immun 61:5140–5146
Parker TS, Levine DM, Chang JC, Laxer J, Coffin CC, Rubin AL (1995) Reconstituted high-density lipoprotein neutralizes gram-negative bacterial lipopolysaccharides in human whole blood. Infect Immun 63:253–258
Takeda K, Akira S (2005) Toll-like receptors in innate immunity. Int Immunol 17:1–14. doi:10.1093/intimm/dxh186
De Nardo D, Labzin LI, Kono H, Seki R, Schmidt SV, Beyer M, Xu D, Zimmer S, Lahrmann C, Schildberg FA, Vogelhuber J, Kraut M, Ulas T, Kerksiek A, Krebs W, Bode N, Grebe A, Fitzgerald ML, Hernandez NJ, Williams BR, Knolle P, Kneilling M, Rocken M, Lutjohann D, Wright SD, Schultze JL, Latz E (2014) High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat Immunol 15:152–160. doi:10.1038/ni.2784
Dandekar A, Qiu Y, Kim H, Wang J, Hou X, Zhang X, Zheng Z, Mendez R, Yu FS, Kumar A, Fang D, Sun F, Zhang K (2016) Toll-like receptor (TLR) signaling interacts with CREBH to modulate high-density lipoprotein (HDL) in response to bacterial endotoxin. J Biol Chem 291:23149–23158. doi:10.1074/jbc.M116.755728
Francone OL, Royer L, Boucher G, Haghpassand M, Freeman A, Brees D, Aiello RJ (2005) Increased cholesterol deposition, expression of scavenger receptors, and response to chemotactic factors in Abca1-deficient macrophages. Arterioscler Thromb Vasc Biol 25:1198–1205. doi:10.1161/01.atv.0000166522.69552.99
Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS (2008) Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem 283:22930–22941. doi:10.1074/jbc.M801408200
Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR (2008) Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation 118:1837–1847. doi:10.1161/circulationaha.108.793869
Baldan A, Gomes AV, Ping P, Edwards PA (2008) Loss of ABCG1 results in chronic pulmonary inflammation. J Immunol 180:3560–3568
Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P (2008) LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 134:97–111. doi:10.1016/j.cell.2008.04.052
Wilhelm AJ, Zabalawi M, Grayson JM, Weant AE, Major AS, Owen J, Bharadwaj M, Walzem R, Chan L, Oka K, Thomas MJ, Sorci-Thomas MG (2009) Apolipoprotein A-I and its role in lymphocyte cholesterol homeostasis and autoimmunity. Arterioscler Thromb Vasc Biol 29:843–849. doi:10.1161/atvbaha.108.183442
Feng H, Guo L, Wang D, Gao H, Hou G, Zheng Z, Ai J, Foreman O, Daugherty A, Li XA (2011) Deficiency of scavenger receptor BI leads to impaired lymphocyte homeostasis and autoimmune disorders in mice. Arterioscler Thromb Vasc Biol 31:2543–2551. doi:10.1161/atvbaha.111.234716
Rueda CM, Rodriguez-Perea AL, Moreno-Fernandez M, Jackson CM, Melchior JT, Davidson WS, Chougnet CA (2017) High density lipoproteins selectively promote the survival of human regulatory T-cells. J Lipid Res. doi:10.1194/jlr.M072835
Pastrana JL, Sha X, Virtue A, Mai J, Cueto R, Lee IA, Wang H, Yang XF (2012) Regulatory T cells and atherosclerosis. J Clin Exp Cardiol 2012:2. doi:10.4172/2155-9880.s12-002
Charles-Schoeman C, Lee YY, Grijalva V, Amjadi S, FitzGerald J, Ranganath VK, Taylor M, McMahon M, Paulus HE, Reddy ST (2012) Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis 71:1157–1162. doi:10.1136/annrheumdis-2011-200493
Field FJ, Watt K, Mathur SN (2010) TNF-alpha decreases ABCA1 expression and attenuates HDL cholesterol efflux in the human intestinal cell line Caco-2. J Lipid Res 51:1407–1415. doi:10.1194/jlr.M002410
Ammirati E, Bozzolo EP, Contri R, Baragetti A, Palini AG, Cianflone D, Banfi M, Uboldi P, Bottoni G, Scotti I, Pirillo A, Grigore L, Garlaschelli K, Monaco C, Catapano AL, Sabbadini MG, Manfredi AA, Norata GD (2014) Cardiometabolic and immune factors associated with increased common carotid artery intima-media thickness and cardiovascular disease in patients with systemic lupus erythematosus. Nutr Metab Cardiovasc Dis 24:751–759. doi:10.1016/j.numecd.2014.01.006
Altruda F, Poli V, Restagno G, Argos P, Cortese R, Silengo L (1985) The primary structure of human hemopexin deduced from cDNA sequence: evidence for internal, repeating homology. Nucleic Acids Res 13:3841–3859
Katnik I, Jadach J (1996) Haptoglobin concentration in serum and other body fluids measured by comparison of its reactivity with hemoglobin and concanavalin A. Arch Immunol Ther Exp (Warsz) 44:45–50
Dobryszycka W (1997) Biological functions of haptoglobin—new pieces to an old puzzle. Eur J Clin Chem Clin Biochem 35:647–654
Buechler C, Ritter M, Orso E, Langmann T, Klucken J, Schmitz G (2000) Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol 67:97–103
Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK (2001) Identification of the haemoglobin scavenger receptor. Nature 409:198–201. doi:10.1038/35051594
Engstrom G, Hedblad B, Tyden P, Lindgarde F (2009) Inflammation-sensitive plasma proteins are associated with increased incidence of heart failure: a population-based cohort study. Atherosclerosis 202:617–622. doi:10.1016/j.atherosclerosis.2008.05.038
Navab M, Anantharamaiah GM, Fogelman AM (2005) The role of high-density lipoprotein in inflammation. Trends Cardiovasc Med 15:158–161. doi:10.1016/j.tcm.2005.05.008
Ye D, Lammers B, Zhao Y, Meurs I, Van Berkel TJ, Van Eck M (2011) ATP-binding cassette transporters A1 and G1, HDL metabolism, cholesterol efflux, and inflammation: important targets for the treatment of atherosclerosis. Curr Drug Targets 12:647–660
Watanabe J, Chou KJ, Liao JC, Miao Y, Meng HH, Ge H, Grijalva V, Hama S, Kozak K, Buga G, Whitelegge JP, Lee TD, Farias-Eisner R, Navab M, Fogelman AM, Reddy ST (2007) Differential association of hemoglobin with proinflammatory high density lipoproteins in atherogenic/hyperlipidemic mice. A novel biomarker of atherosclerosis. J Biol Chem 282:23698–23707. doi:10.1074/jbc.M702163200
Watanabe J, Grijalva V, Hama S, Barbour K, Berger FG, Navab M, Fogelman AM, Reddy ST (2009) Hemoglobin and its scavenger protein haptoglobin associate with apoA-1-containing particles and influence the inflammatory properties and function of high density lipoprotein. J Biol Chem 284:18292–18301. doi:10.1074/jbc.M109.017202
Matuszek MA, Aristoteli LP, Bannon PG, Hendel PN, Hughes CF, Jessup W, Dean RT, Kritharides L (2003) Haptoglobin elutes from human atherosclerotic coronary arteries—a potential marker of arterial pathology. Atherosclerosis 168:389–396
Asleh R, Levy AP (2005) In vivo and in vitro studies establishing haptoglobin as a major susceptibility gene for diabetic vascular disease. Vasc Health Risk Manag 1:19–28
Levy AP, Hochberg I, Jablonski K, Resnick HE, Lee ET, Best L, Howard BV (2002) Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes: the Strong Heart Study. J Am Coll Cardiol 40:1984–1990
Graversen JH, Madsen M, Moestrup SK (2002) CD163: a signal receptor scavenging haptoglobin-hemoglobin complexes from plasma. Int J Biochem Cell Biol 34:309–314
Kaempfer T, Duerst E, Gehrig P, Roschitzki B, Rutishauser D, Grossmann J, Schoedon G, Vallelian F, Schaer DJ (2011) Extracellular hemoglobin polarizes the macrophage proteome toward Hb-clearance, enhanced antioxidant capacity and suppressed HLA class 2 expression. J Proteome Res 10:2397–2408. doi:10.1021/pr101230y
Ross R (1999) Atherosclerosis is an inflammatory disease. Am Heart J 138:S419–S420
Wallberg-Jonsson S, Cvetkovic JT, Sundqvist KG, Lefvert AK, Rantapaa-Dahlqvist S (2002) Activation of the immune system and inflammatory activity in relation to markers of atherothrombotic disease and atherosclerosis in rheumatoid arthritis. J Rheumatol 29:875–882
Morita T (2005) Heme oxygenase and atherosclerosis. Arterioscler Thromb Vasc Biol 25:1786–1795. doi:10.1161/01.atv.0000178169.95781.49
Smeets MB, Pasterkamp G, Lim SK, Velema E, van Middelaar B, de Kleijn DP (2002) Nitric oxide synthesis is involved in arterial haptoglobin expression after sustained flow changes. FEBS Lett 529:221–224
Jahagirdar R, Zhang H, Azhar S, Tobin J, Attwell S, Yu R, Wu J, McLure KG, Hansen HC, Wagner GS, Young PR, Srivastava RA, Wong NC, Johansson J (2014) A novel BET bromodomain inhibitor, RVX-208, shows reduction of atherosclerosis in hyperlipidemic ApoE deficient mice. Atherosclerosis 236:91–100. doi:10.1016/j.atherosclerosis.2014.06.008
Asleh R, Miller-Lotan R, Aviram M, Hayek T, Yulish M, Levy JE, Miller B, Blum S, Milman U, Shapira C, Levy AP (2006) Haptoglobin genotype is a regulator of reverse cholesterol transport in diabetes in vitro and in vivo. Circ Res 99:1419–1425. doi:10.1161/01.res.0000251741.65179.56
Lioupis C, Barbatis C, Drougou A, Koliaraki V, Mamalaki A, Klonaris C, Georgopoulos S, Andrikopoulos V, Bastounis E (2011) Association of haptoglobin genotype and common cardiovascular risk factors with the amount of iron in atherosclerotic carotid plaques. Atherosclerosis 216:131–138. doi:10.1016/j.atherosclerosis.2011.01.028
Purushothaman M, Krishnan P, Purushothaman KR, Baber U, Tarricone A, Perez JS, Wiley J, Kini A, Sharma SK, Fuster V, Moreno PR (2012) Genotype-dependent impairment of hemoglobin clearance increases oxidative and inflammatory response in human diabetic atherosclerosis. Arterioscler Thromb Vasc Biol 32:2769–2775. doi:10.1161/atvbaha.112.252122
Purushothaman KR, Purushothaman M, Levy AP, Lento PA, Evrard S, Kovacic JC, Briley-Saebo KC, Tsimikas S, Witztum JL, Krishnan P, Kini A, Fayad ZA, Fuster V, Sharma SK, Moreno PR (2012) Increased expression of oxidation-specific epitopes and apoptosis are associated with haptoglobin genotype: possible implications for plaque progression in human atherosclerosis. J Am Coll Cardiol 60:112–119. doi:10.1016/j.jacc.2012.04.011
Borrell-Pages M, Romero JC, Juan-Babot O, Badimon L (2011) Wnt pathway activation, cell migration, and lipid uptake is regulated by low-density lipoprotein receptor-related protein 5 in human macrophages. Eur Heart J 32:2841–2850. doi:10.1093/eurheartj/ehr062
Tsaousi A, Williams H, Lyon CA, Taylor V, Swain A, Johnson JL, George SJ (2011) Wnt4/beta-catenin signaling induces VSMC proliferation and is associated with intimal thickening. Circ Res 108:427–436. doi:10.1161/circresaha.110.233999
Srivastava R, Cefalu, AB, Davide, A, Averna MR (2013) A combination of metformin, quercetin, and curcumin restores HDL function and improves atherosclerosis burden in LDLr−/−/ob.ob leptin−/− and LDLr−/− mice by attenuating insulin resistance, hyperglycemia, and low-grade inflammation. ATVB Scientific Session: Abstract
Acknowledgements
The author would like to thank Maurizio Averna, University of Palermo, Palermo, Italy and Charles L Bisgaier, Gemphire Therapeutics, Livonia, MI, USA for many stimulating discussions relating to dysfunctional HDL, diabetes, and atherosclerosis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
During the preparation of this manuscript, the Rai Ajit K. Srivastava served as a consultant to Gemphire Therapeutics and currently employed at Gemphire Therapeutics Inc. The author has no conflict of interest.
Rights and permissions
About this article
Cite this article
Srivastava, R.A.K. Dysfunctional HDL in diabetes mellitus and its role in the pathogenesis of cardiovascular disease. Mol Cell Biochem 440, 167–187 (2018). https://doi.org/10.1007/s11010-017-3165-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3165-z