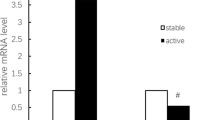
Abstract
CD4+CD28null T cells are present in increased numbers in the peripheral blood of patients with acute coronary syndrome. However, the triggers of expansion of these cells are unclear. Susceptibility to coronary heart disease (CHD) is strongly associated with alleles of human leukocyte antigen (HLA), but it is not equally strong in different human populations. The objective of the study was to investigate association between CD4+CD28null T cells and HLA-DRB1 alleles. The HLA alleles were determined by polymerase chain reaction with sequence-specific primers (PCR-SSP) method, in a group of CHD patients and control subjects from the same area. The number of CD4+CD28null T cells was measured using the flow cytometry technique. The HLA-DRB1*01 (RR = 4.705, P < 0.005) and DRB1*04 (RR = 3.554, P < 0.005) alleles showed the strongest association with CHD in the Chinese population, and increased numbers of CD4+CD28null T cells were found in association with HLA-DRB1*04 (17.1%) and DRB*01 (12.9%), while decreased numbers of CD4+CD28null T cells were present in subjects with DRB1*15 (0.8%). CHD in Chinese population is strongly associated with HLA-DRB1*01 and DRB1*04 haplotypes, and formation of CD4+CD28null T cells was related to HLA-DRB1*01, DRB1*04, and DRB1*15 alleles.


Similar content being viewed by others
References
Davies M (2000) The pathophysiology of acute coronary syndromes. Heart 83:361–366
Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL, Weyand CM (2000) Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation 101:2883–2888
Nakajima T, Schulte S, Warrington KJ, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM (2002) T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation 105:570–575
Shiina T, Inoko H, Kulski JK (2004) An update of the HLA genomic region, locus information and disease associations. Tissue Antigens 64(6):631–649
Farjadian S, Ota M, Inoko H, Ghaderi A (2009) The genetic relationship among Iranian ethnic groups: an anthropological view based on HLA class II gene polymorphism. Mol Biol Rep 36(7):1943–1950
Ayala FJ (1995) The myth of eve: molecular biology and human origins. Science 270:1930–1936
Almawi WY, Busson M, Tamim H, Al-Harbi EM, Finan RR et al (2004) HLA class II profile and distribution of HLA-DRB1 and HLA-DQB1 alleles and haplotypes among Lebanese and Bahraini Arabs. Clin Diagn Lab Immunol 11(4):770–774
Nilsson J, Hansson GK, Shah PK (2005) Immunomodulation of atherosclerosis. Implications for vaccine development. Arterioscler Thromb Vasc Biol 25:18–28
Mathews JD (1975) Ischaemic heart-disease: possible genetic markers. Lancet 2:681–682
Khaitov RM, Polianskaia IS, Alekseev LP (1990) The HLA system antigens in patients with cardiovascular diseases. Ter-Arkh 62:70–74
Dahlen GH, Slunga L, Holmlund G, Lango A, Lindblom B (1993) Lp(a) lipoprotein and HLA-DR genotype in early coronary artery disease. Eur J Immunogenet 20:95–102
Forsblom CM, Sane T, Groop PH, Totterman KJ et al (1999) Risk factors for mortality in tape-II (non-insulin-dependent) idiabetes: evidence of a role for neuropathy and a protective effect of HLA-DR4. Diabetologia 41:1253–1262
Jonasson L, Eriksson T, Dahlen GF et al (1997) Lipoprotein(a) and HLA-DRB1 and -DQB1 gene in coronary artery disease. Atherosclerosis 133:111–114
Zal B, Kaski JC, Arno G, Akiyu JP, Xu Q et al (2004) Heat-shock protein 60-reactive CD4+CD28null T cells in patients with acute coronary syndromes. Circulation 109:1230–1235
Champan A, Stewart SJ, Nepom GT, Green WF et al (1996) CD11b+CD28−CD4+ human T cells activation requirement and association with HLA-DR alleles. J Immunol 157:1780–4771
Acknowledgments
Supported by Heilongjiang Province Nature Science Fund D200907.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, W., Cui, Y., Zhen, L. et al. Association between HLA-DRB1, HLA-DRQB1 alleles, and CD4+CD28null T cells in a Chinese population with coronary heart disease. Mol Biol Rep 38, 1675–1679 (2011). https://doi.org/10.1007/s11033-010-0279-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0279-8