Abstract
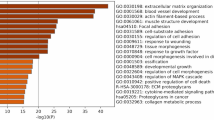
The majority of experimental and clinical studies indicates that the hypertrophied and failing myocardium are characterized by changes in energy and substrate metabolism that attributed to failing heart changes at the genomic level, in fact, heart failure is caused by various diseases, their energy metabolism and substrate are in different genetic variations, then the potential significance of the molecular mechanisms for the aetiology of heart failure is necessary to be evaluated. Persistent viral infection (especially coxsackievirus group B3) of the myocardium in viral myocarditis and viral dilated cardiomyopathy has never been neglected by experts. This study aimed to explore the role and regulatory mechanism of the altered gene expression for energy metabolism involved in mitochondrial oxidative phosphorylation, fatty acid metabolism in viral dilated cardiomyopathy. cDNA Microarray technology was used to evaluate the expression of >35,852 genes in a mice model of viral dilated cardiomyopathy. In total 1385 highly different genes expression, we analyzed 33 altered genes expression for energy metabolism involved in mitochondrial oxidative phosphorylation, fatty acid metabolism and further selected real-time-PCR for quantity one of regulatory mechanisms for energy including fatty acid metabolism—the UCP2 and assayed cytochrome C oxidase activity by Spectrophotometer to explore mitochondrial oxidative phosphorylation function. We found obviously different expression of 33 energy metabolism genes associated with mitochondria oxidative phosphorylation, fatty acid metabolism in cardiomyopathy mouse heart, the regulatory gene for energy metabolism: UCP2 was down-regulated and cytochrome C oxidase activity was decreased. Genes involved in both fatty acid metabolism and mitochondrial oxidative phosphorylation were down-regulated, mitochondrial uncoupling proteins (UCP2) expression did not increase but decrease which might be a kind of adaptive protection response to regulate energy metabolism for ATP produce.



Similar content being viewed by others
References
Lavie CJ, Milani RV, Shah SB et al (2008) Impact of left ventricular geometry on prognosis—a review of Ochsner studies. Ochsner J 8:11–17
Krauser DG, Devereux RB (2006) Ventricular hypertrophy and hypertension: prognostic elements and implications for management. Herz 31:305–316
Beer M, Seyfarth T, Sandstede J, Landschutz W, Lipke C, Kostler H, von Kienlin M, Harre K, Hahn D, Neubauer S (2002) Absolute concentrations of high-energy phosphate metabolites in normal hypertrophied, and failing human myocardium measured noninvasively with (31) P-SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol 40:1267–1274
Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W, Sabbah HN, Hoppel CL (2008) Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res 80(1):30–39
Nojiri H, Shimizu T, Funakoshi M, Yamaguchi O, Zhou H, Kawakami S, Ohta Y, Sami M, Tachibana T, Ishikawa H, Kurosawa H, Kahn RC, Otsu K (2006) Shirasawa T oxidative stress causes heart failure with impaired mitochondrial respiration. J Biol Chem 281:33789–33801
Luptak I, Balschi JA, Xing Y, Leone TC, Kelly DP, Tian R (2005) Decreased contractile and metabolic reserve in peroxisome proliferator-activated receptor-alpha-null hearts can be rescued by increasing glucose transport and utilization. Circulation 112:2339–2346
Neglia D, De Caterina A, Marraccini P, Natali A, Ciardetti M, Vecoli C et al (2007) Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 293:H3270–H3278
Rudiger A, Gasser S, Fischler M et al (2006) Comparable increase of B-type natriuretic peptide and amino-terminal pro-B-type natriuretic peptide levels in patients with severe sepsis, septic shock, and acute heart failure. Crit Care Med 34:2140–2144
Boluyt MO, O’Neill L, Meredith AL, Bing OH, Brooks WW, Conrad CH et al (1994) Alterations in cardiac gene expression during the transition from stable hypertrophy to heart failure. Marked upregulation of genes encoding extracellular matrix components. Circ Res 75:23–32
Jaber WA, Maniu C, Krysiak J, Shapiro BP, Meyer DM, Linke WA, Redfield MM (2008) Titin isoforms, extracellular matrix, and global chamber remodeling in experimental dilated cardiomyopathy: functional implications and mechanistic insight. Circ Heart Fail 1(3):192–199
Schwenk RW, Luiken JJ, Bonen A, Glatz JF (2008) Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc Res 79:249–258
Taha M, Lopaschuk GD (2007) Alterations in energy metabolism in cardiomyopathies. Ann Med 39:594–607
Pecqueur C, Bui T, Gelly C, Hauchard J, Barbot C, Bouillaud F, Ricquier D, Miroux B, Thompson CB (2007) Uncoupling protein-2 controls proliferation by promoting fatty acid oxidation and limiting glycolysis-derived pyruvate utilization. FASEB J 22:9–18
Criscuolo F, del Mar Gonzalez-Barroso M, Le Maho Y, Ricquier D, Bouillaud F (2005) Avian uncoupling protein expressed in yeast mitochondria prevents endogenous free radical damage. Proc Biol Sci 272:803–810
Chapman NM, Kim KS (2008) Persistent coxsackievirus infection: enterovirus persistence in chronic myocarditis and dilated cardiomyopathy. Curr Top Microbiol Immunol 323:275–292
Tam PE (2006) Coxsackievirus myocarditis: interplay between virus and host in the pathogenesis of heart disease. Viral Immunol 19:133–146
Marin Garcia J, Goldenthal MJ (2002) Understanding the impact of mitochondrial defects inca rdiovascular disease: a review. J Card Fail 8(5):347–360
Smith DN (1994) Bioimpedance measurement of cardiac output [J]. Crit Care Med 22(9):1513–1515
Prabu SK, Raza H, Srinivasan S, Spear JF, Avadhani NG (2006) Protein kinase A-mediated phosphorylation modulates cytochrome c oxidase function and augments hypoxia and myocardial ischemia-related injury. J Biol Chem 281:2061–2070
Mutch DM, Berger A, Mansourian R, Rytz A, Roberts MA (2002) The limit fold change model: a practical approach for selecting differentially expressed genes from microarray data. BMC Bioinformatics 3:17
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:E45
Tichopad A, Dilger M, Schwarz G, Pfaffl MW (2003) Standardized determination of real-time PCR efficiency from a single reaction set-up. Nucleic Acids Res 31(20):E122
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM et al (2000) Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25:25–29
Sharov VG, Todor AV, Silverman N, Goldstein S, Sabbah HN (2000) Abnormal mitochondrial respiration in failed human myocardium. J Mol Cell Cardiol 32:2361–2367
Esposito LA, Melov S, Panov A, Cottrell BA, Wallace DC (1999) Mitochondrial disease in mouse results in increased oxidative stress. Proc Natl Acad Sci USA 96:4820–4825
Huang G, Lu H, Hao A, Ng DC, Ponniah S, Guo K, Lufei C, Zeng Q, Cao X (2004) GRIM-19, a cell death regulatory protein, is essential for assembly and function of mitochondrial complex I. Mol Cell Biol 24:8447–8456
Lemeshko VV, Lemeshko SV (2004) The voltage-dependent anion channel as a biological transistor: theoretical considerations. Eur Biophys J 33(4):352–359
Santosh S, Pawan K, Karpagam P et al (2006) Defect in oxidative phosphorylation in LV papillary muscle mitochondria of patients undergoing mitral valve replacement. Mitochondrion 6(2):89–93
Nubel T, Emre Y, Rabier D, Chadefaux B, Ricquier D, Bouillaud F (2008) Modified glutamine catabolism in macrophages of Ucp2 knock-out mice. Biochim Biophys Acta 1777:48–54
Noma T, Nishiyama A, Mizushige K et al (2001) Possible role of uncoupling protein in regulation of myocardial energy metabolism in aortic regurgitation model rats. FASEB J 15:1206–1208
Razeghi P, Young ME, Cockrill TC, Frazier OH, Taegtmeyer H (2002) Down-regulation of myocardial myocyte enhancer factor 2C and myocyte enhancer factor 2C-regulated gene expression in diabetic patients with non-ischemic heart failure. Circulation 106:407–411
Acknowledgment
This work has been funded by the Natural Science Foundation of Heilongjiang Province, China (Contract No. D200982), Education Department projects of Heilongjiang, China (Contract No. 1154G08).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jing Xu and Hong-gang Nie contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xu, J., Nie, Hg., Zhang, Xd. et al. Down-regulated energy metabolism genes associated with mitochondria oxidative phosphorylation and fatty acid metabolism in viral cardiomyopathy mouse heart. Mol Biol Rep 38, 4007–4013 (2011). https://doi.org/10.1007/s11033-010-0519-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0519-y