Abstract
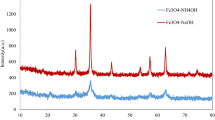
The production of monodispersed magnetic nanoparticles with appropriate surface modification has attracted increasing attention in biomedical applications including drug delivery, separation, and purification of biomolecules from the matrices. In the present study, we report rapid and room temperature reaction synthesis of gold-coated iron nanoparticles in aqueous solution using the borohydride reduction of HAuCl4 under sonication for the first time. The resulting nanoparticles were characterized with transmission electron microscopy (TEM), electron spectroscopy for chemical analysis (ESCA), ultraviolet visible spectroscopy (UV–Vis), and X-ray diffraction (XRD). Surface charges and magnetic properties of the nanoparticles were also examined. The pattern of Fe3O4 nanoparticles is face centered cubic with an average diameter of 9.5 nm and the initial reduction of gold on the surface of Fe3O4 particles exhibits uniform Fe3O4–Au nanoparticles with an average diameter of 12.5 nm. The saturation magnetization values for the uncoated and gold-coated Fe3O4 nanoparticles were found to be 30 and 4.5 emu/g, respectively, at 300 K. The progression of binding events between boronic acid terminated ligand shell and fructose based on the covalent bonding interaction was measured by absorbance spectral changes. Immunomagnetic separation was also performed at different E. coli concentration to evaluate capturing efficiency of resulting nanoparticles. Immunomagnetic separation percentages were varied in a range of 52.1 and 21.9% depend on the initial bacteria counts.







Similar content being viewed by others
References
Ban Z, Barnaov YA, Li F, Golup VO, O’Conner CJ (2005) The synthesis of core shell iron@gold nanoparticles and their characterization. J Mater Chem 15:4660–4662
Brown LO, Hutchison JE (1997) Convenient preparation of stable, narrow-dispersity, gold nanocrystals by ligand exchange reactions. J Am Chem Soc 119(50):12384–12385
Brown LO, Hutchison JE (1999) Controlled growth of gold nanoparticles during ligand exchange. J Am Chem Soc 121(4):882–883
Carpenter EE (2001) Iron nanoparticles as potential magnetic carriers. J Magn Magn Mater 225(1–2):17–20
Carpenter EE, Kumbhar A, Wiemann JA, Srikanth H, Wiggins J, Zhou W et al (2000) Synthesis and magnetic properties of gold-iron-gold nanocomposites. Mater Sci Eng A 286(1):81–86
Chamberlin RV, Humfeld KD, Farrell D, Yamamuro S, Ijiri Y, Majetich SA (2002) Magnetic relaxation of iron nanoparticles. J Appl Phys 91(10):6961–6963
Chen M, Nikles DE (1999) Chain-of-cubes iron nanoparticles prepared by borohydride reduction of acicular akaganeite particles. J Appl Phys 85(8):5504–5506
Chen M, Yamamuro S, Farrell D, Majetich SA (2003) Gold-coated iron nanoparticles for biomedical applications. J Appl Phys 93(10):7551–7553
Chilkoti A, Stayton PS (1995) Molecular-origins of the slow streptavidin-biotin dissociation kinetics. J Am Chem Soc 117(43):10622–10628
Cho SJ, Idrobo JC, Olamit J, Liu K, Browning ND, Kauzlarich SM (2005) Growth mechanisms and oxidation resistance of gold-coated iron nanoparticles. Chem Mater 17(12):3181–3186
Chouly C, Pouliquen D, Lucet I, Jeune JJ, Jallet P (1996) Development of superparamagnetic nanoparticles for MRI: effect of particle size, charge and surface nature on biodistribution. J Microencapsul 13(3):245–255
Gu HW, Zheng R, Zhang X, Xu B (2004) Facile one-pot synthesis of bifunctional heterodimers of nanoparticles: a conjugate of quantum dot and magnetic nanoparticles. J Am Chem Soc 126(18):5664–5665
Gupta AK, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26(18):3995–4021
Heitsch AT, Smith DK, Patel RN, Ress D, Korgel BA (2008) Multifunctional particles: magnetic nanocrystals and gold nanorods coated with fluorescent dye-doped silica shells. J Solid State Chem 181(7):1590–1599
Hosseinkhani H (2006) DNA nanoparticles for gene delivery to cells and tissue. Int J Nanotechnol 3(4):416–461
Hosseinkhani H, Hosseinkhani M (2009) Biodegradable polymer-metal complexes for gene and drug delivery. Curr Drug Saf 4(1):79–83
Hosseinkhani H, Tabata Y (2006) Self assembly of DNA nanoparticles with polycations for the delivery of genetic materials into cells. J Nanosci Nanotechnol 6(8):2320–2328
Hosseinkhani H, Aoyamaa T, Ogawab O, Tabataa Y (2003) Tumor targeting of gene expression through metal-coordinated conjugation with dextran. J Control Release 88:297–312
Hosseinkhani H, Hosseinkhani M, Gabrielson NP, Pack DW, Khademhosseini A, Kobayashi H (2008) DNA nanoparticles encapsulated in 3D tissue-engineered scaffolds enhance osteogenic differentiation of mesenchymal stem cells. J Biomed Mater Res A 85:47–60
Jeong J, Ha TH, Chung BH (2006) Enhanced reusability of hexa-arginine-tagged esterase immobilized on gold-coated magnetic nanoparticles. Anal Chim Acta 569(1–2):203–209
Kalinin NL, Ward LD, Winzor DJ (1995) Effects of solute multivalence on the evaluation of binding constants by biosensor technology-studies with concanavalin-a and interleukin-6 as partitioning proteins. J Anal Biochem 228(2):238–244
Lee S, Perez Luna VH (2005) Dextran-gold nanoparticle hybrid material for biomolecule immobilization and detection. Anal Chem 77(22):7204–7211
Lee JH, Huh YM, Jun YW, Seo JW, Jang JT, Song HT et al (2007) Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med 13(1):95–99
Lin J, Zhou W, Kumbhar A, Wiemann J, Fang J, Carpenter EE et al (2001) Gold-coated iron (Fe@Au) nanoparticles: synthesis, characterization, and magnetic field-induced self-assembly. J Solid State Chem 159(1):26–31
Lu Y, Yin YD, Mayers BT, Xia YN (2002) Modifying the surface properties of superparamagnetic iron oxide nanoparticles through a sol-gel approach. Nano Lett 2(3):183–186
Lyon JL, Fleming DA, Stone MB, Schiffer P, Williams ME (2004) Synthesis of Fe oxide core/Au shell nanoparticles by iterative hydroxylamine seeding. Nano Lett 4(4):719–723
Mader HS, Wolfbeis OS (2008) Boronic acid based probes for microdetermination of saccharides and glycosylated biomolecules. Microchim Acta 162(1–2):1–34
Mandal M, Kundu S, Ghosh SK, Panigrahi S, Sau TK, Yusuf SM et al (2005) Magnetite nanoparticles with tunable gold or silver shell. J Colloid Interface Sci 286(1):187–194
Meldrum FC, Heywood BR, Mann S (1992) Magnetoferritin—in vitro synthesis of a novel magnetic protein. Science 257(5069):522–523
Mikhaylova M, Kim DK, Bobrysheva N, Osmolowsky M, Semenov V, Tsakalatos T et al (2004) Superparamagnetism of magnetite nanoparticles: dependence on surface modification. Langmuir 20(6):2472–2477
Pham TTH, Cao C, Sim SJ (2008) Application of citrate-stabilized gold coated ferric oxide composite nanoparticles for biological separations. J Magn Magn Mater 320:2049–2055
Seo WS, Lee JH, Sun XM, Suzuki Y, Mann D, Liu Z, Terashima M, Yang PC, Mcconnell MV, Niohimura DG, Dai HJ (2006) FeCo/graphitic-shell nanocrystals as advanced magnetic-resonance-imaging and near-infrared agents. Nat Mater 5(12):971–976
Storm G, Belliot SO, Daemen T, Lasic DD (1995) Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Deliv Rev 17(1):31–48
Stuart DA, Haes AJ, Yonzon CR, Hicks EM, Van Duyne RP (2005) Biological applications of localised surface plasmonic phenomenae. IEE Proc Nanobiotechnol 152(1):13–32
Subramani K, Hosseinkhani H, Khraisat A, Hosseinkhani M, Pathak Y (2009) Targeting nanoparticles as drug delivery systems for cancer treatment. Curr Nanosci 5(2):134–140
Sun SH, Murray CB, Weller D, Folks L, Moser A (2000) Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 287(5460):1989–1992
Tanaka T, Matsunaya T (2000) Fully automated chemiluminescence immunoassay of insulin using antibody-protein A-bacterial magnetic particle complexes. Anal Chem 72(15):3518–3522
Ugelstad J, Mork PC, Kaggerud KH, Ellingsen T, Berge A (1980) Swelling of oligomer-polymer particles. New methods of preparation of emulsions and polymer dispersions. Adv Colloid Interface Sci 13(1–2):101–140
Wang LY, Luo J, Maye MM, Fan Q, Rendeng Q, Engelhard MH et al (2005a) Iron oxide-gold core-shell nanoparticles and thin film assembly. J Mater Chem 15(18):1821–1832
Wang LY, Luo J, Fan Q, Suzuki M, Suzuki IS, Engelhard MH, Lin YH, Kim N, Wang JQ, Zhong CJ (2005b) Monodispersed core-shell Fe3O4@Au nanoparticles. J Phys Chem B 109(46):21593–21601
Warner MG, Reed SM, Hutchision JE (2000) Small, water-soluble, ligand-stabilized gold nanoparticles synthesized by interfacial ligand exchange reactions. Chem Mater 12(11):3316–3320
Weare WW, Reed SM, Warner MG, Hutchison JE (2000) Improved synthesis of small (d(CORE) approximate to 1.5 nm) phosphine-stabilized gold nanoparticles. J Am Chem Soc 122(51):12890–12891
Weissledel R, Moure A, Mahmood U, Bhorade R, Benveniste M, Chiocca E et al (2000) In vivo magnetic resonance imaging of transgene expression. Nat Med 6(3):351–355
Widder KJ, Senge AE, Ranney DF (1980) Invitro release of biologically-active adriamycin by magnetically responsive albumin microspheres. Cancer Res 40(10):3512–3517
Xu H, Cui L, Tong N, Gu H (2006) Development of high magnetization Fe3O4/polystyrene/silica nanospheres via combined miniemulsion/emulsion polymerization. J Am Chem Soc 128(49):15582–15583
Xu Z, Hou Y, Sun S (2007) Magnetic core/shell Fe3O4/Au and Fe3O4/Au/Ag nanoparticles with tunable plasmonic properties. J Am Chem Soc 129:8698–8699
Xu C, Xie J, Ho D, Wang C, Kohler N, Walsh EG, Morgan JR, Chin YE, Sun S (2008) Au-Fe3O4 Dumbbell nanoparticles as dual functional probes. Angew Chem Int Ed 47:173–176
Yamazaki M, Ito M (1990) Deformation and instability in membrane-structure of phospholipid-vesicles caused by osmophobic association mechanical-stress model for the mechanism of poly(ethylene glycol)-induced membrane-fusion. Biochemistry 29(5):1309–1314
Yonezawa T, Yasui K, Kimizuka N (2001) Controlled formation of smaller gold nanoparticles by the use of four-chained disulfide stabilizer. Langmuir 17(2):271–273
Zhang Y, Kohler N, Zhang MQ (2002) Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials 23(7):1553–1561
Zhao MQ, Sun L, Crooks RM (1998) Preparation of Cu nanoclusters within dendrimer templates. J Am Chem Soc 120(19):4877–4878
Zhou WL, Carpenter EE, Lin J, Kumbhar A, Sims J, O’Conner CJ (2001) Nanostructures of gold coated iron core-shell nanoparticles and the nanobands assembled under magnetic field. Eur Phys J D 16(1–3):289–292
Acknowledgment
The authors are grateful for the financial supports provided by The Scientific and Technological Research Council of Turkey; Project Number: 107T682.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamer, U., Gündoğdu, Y., Boyacı, İ.H. et al. Synthesis of magnetic core–shell Fe3O4–Au nanoparticle for biomolecule immobilization and detection. J Nanopart Res 12, 1187–1196 (2010). https://doi.org/10.1007/s11051-009-9749-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-009-9749-0