Abstract
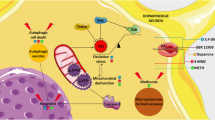
Dopamine (DA) and its metabolites containing two hydroxyl residues exert cytotoxicity in dopaminergic neuronal cells, primarily due to the generation of highly reactive DA and DOPA quinones. Quinone formation is closely linked to other representative hypotheses such as mitochondrial dysfunction, inflammation, oxidative stress, and dysfunction of the ubiquitin-proteasome system, in the pathogenesis of neurodegenerative diseases such as Parkinson’s disease and methamphetamine-induced neurotoxicity. Therefore, pathogenic effects of the DA quinone have focused on dopaminergic neuron-specific oxidative stress. Recently, various studies have demonstrated that some intrinsic molecules and several drugs exert protective effects against DA quinone-induced damage of dopaminergic neurons. In this article, we review recent studies on some neuroprotective approaches against DA quinone-induced dysfunction and/or degeneration of dopaminergic neurons.

Similar content being viewed by others
References
Asanuma M, Miyazaki I, Ogawa N (2003) Dopamine- or l-DOPA-induced neurotoxicity: the role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease. Neurotox Res 5:165–176
Asanuma M, Miyazaki I, Diaz-Corrales FJ, Ogawa N (2004) Quinone formation as dopaminergic neuron-specific oxidative stress in pathogenesis of sporadic Parkinson’s disease and neurotoxin-induced parkinsonism. Acta Med Okayama 58:221–233
Choi HJ, Kim SW, Lee SY, Hwang O (2003) Dopamine-dependent cytotoxicity of tetrahydrobiopterin: a possible mechanism for selective neurodegeneration in Parkinson’s disease. J Neurochem 86:143–152. doi:10.1046/j.1471-4159.2003.01808.x
Choi HJ, Lee SY, Cho Y, Hwang O (2005) Inhibition of vesicular monoamine transporter enhances vulnerability of dopaminergic cells: relevance to Parkinson’s disease. Neurochem Int 46:329–335. doi:10.1016/j.neuint.2004.10.009
LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ (2005) Dopamine covalently modifies and functionally inactivates parkin. Nat Med 11:1214–1221. doi:10.1038/nm1314
Graham DG (1978) Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol 14:633–643
Tse DC, McCreery RL, Adams RN (1976) Potential oxidative pathways of brain catecholamines. J Med Chem 19:37–40. doi:10.1021/jm00223a008
Miyazaki I, Asanuma M, Diaz-Corrales FJ, Miyoshi K, Ogawa N (2005) Dopamine agonist pergolide prevents levodopa-induced quinoprotein formation in parkinsonian striatum and shows quenching effects on dopamine-semiquinone generated in vitro. Clin Neuropharmacol 28:155–160. doi:10.1097/01.wnf.0000175523.33334.24
Miyazaki I, Asanuma M, Diaz-Corrales FJ, Fukuda M, Kitaichi K, Miyoshi K et al (2006) Methamphetamine-induced dopaminergic neurotoxicity is regulated by quinone formation-related molecules. FASEB J 20:571–573
Berman SB, Hastings TG (1999) Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson’s disease. J Neurochem 73:1127–1137. doi:10.1046/j.1471-4159.1999.0731127.x
Kuhn DM, Francescutti-Verbeem DM, Thomas DM (2006) Dopamine quinones activate microglia and induce a neurotoxic gene expression profile: relationship to methamphetamine-induced nerve ending damage. Ann NY Acad Sci 1074:31–41. doi:10.1196/annals.1369.003
Li H, Dryhurst G (1997) Irreversible inhibition of mitochondrial complex I by 7-(2-aminoethyl)-3, 4-dihydro-5-hydroxy-2H-1, 4-benzothiazine-3-carboxylic acid (DHBT-1): a putative nigral endotoxin of relevance to Parkinson’s disease. J Neurochem 69:1530–1541
Zafar KS, Siegel D, Ross D (2006) A potential role for cyclized quinones derived from dopamine, DOPA, and 3, 4-dihydroxyphenylacetic acid in proteasomal inhibition. Mol Pharmacol 70:1079–1086. doi:10.1124/mol.106.024703
Hastings TG (1995) Enzymatic oxidation of dopamine: the role of prostaglandin H synthase. J Neurochem 64:919–924
Korytowski W, Sarna T, Kalyanaraman B, Sealy RC (1987) Tyrosinase-catalyzed oxidation of dopa and related catechol(amine)s: a kinetic electron spin resonance investigation using spin-stabilization and spin label oximetry. Biochim Biophys Acta 924:383–392
Foppoli C, Coccia R, Cini C, Rosei MA (1997) Catecholamines oxidation by xanthine oxidase. Biochim Biophys Acta 1334:200–206
Rosei MA, Blarzino C, Foppoli C, Mosca L, Coccia R (1994) Lipoxygenase-catalyzed oxidation of catecholamines. Biochem Biophys Res Commun 200:344–350. doi:10.1006/bbrc.1994.1454
Fornstedt B, Rosengren E, Carlsson A (1986) Occurrence and distribution of 5-S-cysteinyl derivatives of dopamine, dopa and dopac in the brains of eight mammalian species. Neuropharmacology 25:451–454. doi:10.1016/0028-3908(86)90242-X
Ito S, Fujita K (1982) Conjugation of dopa and 5-S-cysteinyldopa with cysteine mediated by superoxide radical. Biochem Pharmacol 31:2887–2889. doi:10.1016/0006-2952(82)90161-7
Fornai F, Lenzi P, Gesi M, Ferrucci M, Lazzeri G, Busceti CL et al (2003) Fine structure and biochemical mechanisms underlying nigrostriatal inclusions and cell death after proteasome inhibition. J Neurosci 23:8955–8966
Yoshimoto Y, Nakaso K, Nakashima K (2005) l-DOPA and dopamine enhance the formation of aggregates under proteasome inhibition in PC12 cells. FEBS Lett 579:1197–1202. doi:10.1016/j.febslet.2004.12.091
Keller JN, Huang FF, Dimayuga ER, Maragos WF (2000) Dopamine induces proteasome inhibition in neural PC12 cell line. Free Radic Biol Med 29:1037–1042. doi:10.1016/S0891-5849(00)00412-3
Kuhn DM, Arthur RE Jr, Thomas DM, Elferink LA (1999) Tyrosine hydroxylase is inactivated by catechol-quinones and converted to a redox-cycling quinoprotein: possible relevance to Parkinson’s disease. J Neurochem 73:1309–1317. doi:10.1046/j.1471-4159.1999.0731309.x
Xu Y, Stokes AH, Roskoski RJ, Vrana KE (1998) Dopamine, in the presence of tyrosinase, covalently modifies and inactivates tyrosine hydroxylase. J Neurosci Res 54:691–697. doi :10.1002/(SICI)1097-4547(19981201)54:5<691::AID-JNR14>3.0.CO;2-F
Whitehead RE, Ferrer JV, Javitch JA, Justice JB (2001) Reaction of oxidized dopamine with endogenous cysteine residues in the human dopamine transporter. J Neurochem 76:1242–1251. doi:10.1046/j.1471-4159.2001.00125.x
Machida Y, Chiba T, Takayanagi A, Tanaka Y, Asanuma M, Ogawa N et al (2005) Common anti-apoptotic roles of parkin and alpha-synuclein in human dopaminergic cells. Biochem Biophys Res Commun 332:233–240. doi:10.1016/j.bbrc.2005.04.124
Conway KA, Rochet JC, Bieganski RM, Lansbury PT Jr (2001) Kinetic stabilization of the α-synuclein protofibril by a dopamine-α-synuclein adduct. Science 294:1346–1349. doi:10.1126/science.1063522
Goldberg MS, Lansbury PT Jr, Helfand SL (2000) Is there a cause-and-effect relationship between alpha-synuclein fibrillization and Parkinson’s disease? Nat Cell Biol 2:E115–E119. doi:10.1038/35041081
Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT Jr (2000) Acceleration of oligomerization, not fibrillization, is a shared property of both α-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc Natl Acad Sci USA 97:571–576. doi:10.1073/pnas.97.2.571
Volles MJ, Lee SJ, Rochet JC, Shtilerman MD, Ding TT, Kessler JC et al (2001) Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson’s disease. Biochemistry 40:7812–7819. doi:10.1021/bi0102398
Kuhn DM, Arthur R Jr (1998) Dopamine inactivates tryptophan hydroxylase and forms a redox-cycling quinoprotein: possible endogenous toxin to serotonin neurons. J Neurosci 18:7111–7117
Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A et al (2004) Levodopa and the progression of Parkinson’s disease. N Engl J Med 351:2498–2508. doi:10.1056/NEJMoa033447
Asanuma M, Miyazaki I, Diaz-Corrales FJ, Shimizu M, Tanaka K, Ogawa N (2005) Pramipexole has ameliorating effects on levodopa-induced abnormal dopamine turnover in parkinsonian striatum and quenching effects on dopamine-semiquinone generated in vitro. Neurol Res 27:533–539. doi:10.1179/016164105X22093
Ogawa N, Tanaka K, Asanuma M (2000) Bromocriptine markedly suppresses levodopa-induced abnormal increase of dopamine turnover in the parkinsonian striatum. Neurochem Res 25:755–758. doi:10.1023/A:1007530720544
Cadet JL, Brannock C (1998) Free radicals and the pathobiology of brain dopamine systems. Neurochem Int 32:117–131. doi:10.1016/S0197-0186(97)00031-4
Cadet JL, Jayanthi S, Deng X (2003) Speed kills: cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. FASEB J 17:1775–1788. doi:10.1096/fj.03-0073rev
Kita T, Wagner GC, Nakashima T (2003) Current research on methamphetamine-induced neurotoxicity: animal models of monoamine disruption. J Pharmacol Sci 92:178–195. doi:10.1254/jphs.92.178
Cubells JF, Rayport S, Rajendran G, Sulzer D (1994) Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci 14:2260–2271
Fumagalli F, Gainetdinov RR, Wang YM, Valenzano KJ, Miller GW, Caron MG (1999) Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J Neurosci 19:2424–2431
LaVoie MJ, Hastings TG (1999) Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci 19:1484–1491
Emdadul Haque M, Asanuma M, Higashi Y, Miyazaki I, Tanaka K, Ogawa N (2003) Apoptosis-inducing neurotoxicity of dopamine and its metabolites via reactive quinone generation in neuroblastoma cells. Biochim Biophys Acta 1619:39–52
Haque ME, Asanuma M, Higashi Y, Miyazaki I, Tanaka K, Ogawa N (2003) Overexpression of Cu-Zn superoxide dismutase protects neuroblastoma cells against dopamine cytotoxicity accompanied by increase in their glutathione level. Neurosci Res 47:31–37. doi:10.1016/S0168-0102(03)00166-4
Lai CT, Yu PH (1997) Dopamine- and l-beta-3, 4-dihydroxyphenylalanine hydrochloride (l-DOPA)-induced cytotoxicity towards catecholaminergic neuroblastoma SH-SY5Y cells. Effects of oxidative stress and antioxidative factors. Biochem Pharmacol 53:363–372. doi:10.1016/S0006-2952(96)00731-9
Offen D, Ziv I, Sternin H, Melamed E, Hochman A (1996) Prevention of dopamine-induced cell death by thiol antioxidants: possible implications for treatment of Parkinson’s disease. Exp Neurol 141:32–39. doi:10.1006/exnr.1996.0136
Chinta SJ, Andersen JK (2006) Reversible inhibition of mitochondrial complex I activity following chronic dopaminergic glutathione depletion in vitro: implications for Parkinson’s disease. Free Radic Biol Med 41:1442–1448. doi:10.1016/j.freeradbiomed.2006.08.002
Chinta SJ, Kumar MJ, Hsu M, Rajagopalan S, Kaur D, Rane A et al (2007) Inducible alterations of glutathione levels in adult dopaminergic midbrain neurons result in nigrostriatal degeneration. J Neurosci 27:13997–14006. doi:10.1523/JNEUROSCI.3885-07.2007
Solano RM, Casarejos MJ, Menendez-Cuervo J, Rodriguez-Navarro JA, Garcia de Yebenes J, Mena MA (2008) Glial dysfunction in parkin null mice: effects of aging. J Neurosci 28:598–611. doi:10.1523/JNEUROSCI.4609-07.2008
Penkowa M (2006) Metallothioneins are multipurpose neuroprotectants during brain pathology. FEBS J 273:1857–1870. doi:10.1111/j.1742-4658.2006.05207.x
Xie T, Tong L, McCann UD, Yuan J, Becker KG, Mechan AO et al (2004) Identification and characterization of metallothionein-1 and -2 gene expression in the context of (+/−)3, 4-methylenedioxymethamphetamine-induced toxicity to brain dopaminergic neurons. J Neurosci 24:7043–7050. doi:10.1523/JNEUROSCI.1626-04.2004
Aschner M (1998) Metallothionein (MT) isoforms in the central nervous system (CNS): regional and cell-specific distribution and potential functions as an antioxidant. Neurotoxicology 19:653–660
Penkowa M, Carrasco J, Giralt M, Moos T, Hidalgo J (1999) CNS wound healing is severely depressed in metallothionein I- and II-deficient mice. J Neurosci 19:2535–2545
Miura T, Muraoka S, Ogiso T (1997) Antioxidant activity of metallothionein compared with reduced glutathione. Life Sci 60:PL301–309
Hussain S, Slikker W Jr, Ali SF (1996) Role of metallothionein and other antioxidants in scavenging superoxide radicals and their possible role in neuroprotection. Neurochem Int 29:145–152. doi:10.1016/0197-0186(95)00114-X
Ebadi M, Ramana Kumari MV, Hiramatsu M, Hao R, Pfeiffer RF, Rojas P (1998) Metallothionein, neurotrophins and selegiline in providing neuroprotection in Parkinson’s disease. Restor Neurol Neurosci 12:103–111
Sato M, Bremner I (1993) Oxygen free radicals and metallothionein. Free Radic Biol Med 14:325–337. doi:10.1016/0891-5849(93)90029-T
Miyazaki I, Asanuma M, Hozumi H, Miyoshi K, Sogawa N (2007) Protective effects of metallothionein against dopamine quinone-induced dopaminergic neurotoxicity. FEBS Lett 581:5003–5008. doi:10.1016/j.febslet.2007.09.046
Cadenas E, Mira D, Brunmark A, Lind C, Segura-Aguilar J, Ernster L (1988) Effect of superoxide dismutase on the autoxidation of various hydroquinones—a possible role of superoxide dismutase as a superoxide:semiquinone oxidoreductase. Free Radic Biol Med 5:71–79. doi:10.1016/0891-5849(88)90032-9
Hara H, Ohta M, Ohta K, Kuno S, Adachi T (2003) Increase of antioxidative potential by tert-butylhydroquinone protects against cell death associated with 6-hydroxydopamine-induced oxidative stress in neuroblastoma SH-SY5Y cells. Brain Res Mol Brain Res 119:125–131. doi:10.1016/j.molbrainres.2003.08.021
Munday R, Smith BL, Munday CM (1998) Effects of butylated hydroxyanisole and dicoumarol on the toxicity of menadione to rats. Chem Biol Interact 108:155–170. doi:10.1016/S0009-2797(97)00105-1
Duffy S, So A, Murphy TH (1998) Activation of endogenous antioxidant defenses in neuronal cells prevents free radical-mediated damage. J Neurochem 71:69–77
Zafar KS, Inayat-Hussain SH, Siegel D, Bao A, Shieh B, Ross D (2006) Overexpression of NQO1 protects human SK-N-MC neuroblastoma cells against dopamine-induced cell death. Toxicol Lett 166:261–267. doi:10.1016/j.toxlet.2006.07.340
Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA (2003) Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem 278:12029–12038. doi:10.1074/jbc.M211558200
Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD et al (1999) Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13:76–86. doi:10.1101/gad.13.1.76
Kang MI, Kobayashi A, Wakabayashi N, Kim SG, Yamamoto M (2004) Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc Natl Acad Sci USA 101:2046–2051. doi:10.1073/pnas.0308347100
Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M et al (2004) Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci USA 101:2040–2045. doi:10.1073/pnas.0307301101
Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y et al (2002) Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA 99:11908–11913. doi:10.1073/pnas.172398899
Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP (2006) DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci USA 103:15091–15096. doi:10.1073/pnas.0607260103
Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE (2000) Inflammatory mechanisms in Alzheimer’s disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J Neurosci 20:558–567
Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB (2004) Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J Neurochem 88:494–501
Kielian T, Drew PD (2003) Effects of peroxisome proliferator-activated receptor-gamma agonists on central nervous system inflammation. J Neurosci Res 71:315–325. doi:10.1002/jnr.10501
Kim EH, Surh YJ (2006) 15-deoxy-Delta12, 4-prostaglandin J2 as a potential endogenous regulator of redox-sensitive transcription factors. Biochem Pharmacol 72:1516–1528. doi:10.1016/j.bcp.2006.07.030
Hearing VJ, Ekel TM (1976) Mammalian tyrosinase. A comparison of tyrosine hydroxylation and melanin formation. Biochem J 157:549–557
Miranda M, Botti D (1983) Harding-passey mouse-melanoma tyrosinase inactivation by reaction products and activation by l-epinephrine. Gen Pharmacol 14:231–237. doi:10.1016/0306-3623(83)90002-2
Hasegawa T, Matsuzaki M, Takeda A, Kikuchi A, Furukawa K, Shibahara S et al (2003) Increased dopamine and its metabolites in SH-SY5Y neuroblastoma cells that express tyrosinase. J Neurochem 87:470–475. doi:10.1046/j.1471-4159.2003.02008.x
Higashi Y, Asanuma M, Miyazaki I, Ogawa N (2000) Inhibition of tyrosinase reduces cell viability in catecholaminergic neuronal cells. J Neurochem 75:1771–1774. doi:10.1046/j.1471-4159.2000.0751771.x
Mattammal MB, Strong R, Lakshmi VM, Chung HD, Stephenson AH (1995) Prostaglandin H synthetase-mediated metabolism of dopamine: implication for Parkinson’s disease. J Neurochem 64:1645–1654
Ferger B, Teismann P, Earl CD, Kuschinsky K, Oertel WH (1999) Salicylate protects against MPTP-induced impairments in dopaminergic neurotransmission at the striatal and nigral level in mice. Naunyn Schmiedebergs Arch Pharmacol 360:256–261. doi:10.1007/s002109900079
Aubin N, Curet O, Deffois A, Carter C (1998) Aspirin and salicylate protect against MPTP-induced dopamine depletion in mice. J Neurochem 71:1635–1642
Teismann P, Ferger B (2001) Inhibition of the cyclooxygenase isoenzymes COX-1 and COX-2 provide neuroprotection in the MPTP-mouse model of Parkinson’s disease. Synapse 39:167–174. doi :10.1002/1098-2396(200102)39:2<167::AID-SYN8>3.0.CO;2-U
Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S et al (2003) Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc Natl Acad Sci USA 100:5473–5478. doi:10.1073/pnas.0837397100
Asanuma M, Tsuji T, Miyazaki I, Miyoshi K, Ogawa N (2003) Methamphetamine-induced neurotoxicity in mouse brain is attenuated by ketoprofen, a non-steroidal anti-inflammatory drug. Neurosci Lett 352:13–16. doi:10.1016/j.neulet.2003.08.015
Chae SW, Bang YJ, Kim KM, Lee KY, Kang BY, Kim EM et al (2007) Role of cyclooxygenase-2 in tetrahydrobiopterin-induced dopamine oxidation. Biochem Biophys Res Commun 359:735–741. doi:10.1016/j.bbrc.2007.05.190
Asanuma M, Miyazaki I, Ogawa N (2004) Neuroprotective effects of nonsteroidal anti-inflammatory drugs on neurodegenerative diseases. Curr Pharm Des 10:695–700. doi:10.2174/1381612043453072
Jaradat MS, Wongsud B, Phornchirasilp S, Rangwala SM, Shams G, Sutton M et al (2001) Activation of peroxisome proliferator-activated receptor isoforms and inhibition of prostaglandin H(2) synthases by ibuprofen, naproxen, and indomethacin. Biochem Pharmacol 62:1587–1595. doi:10.1016/S0006-2952(01)00822-X
Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA (1997) Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem 272:3406–3410. doi:10.1074/jbc.272.6.3406
Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B et al (2000) Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J Neurosci 20:5709–5714
Tanaka K, Miyazaki I, Fujita N, Haque ME, Asanuma M, Ogawa N (2001) Molecular mechanism in activation of glutathione system by ropinirole, a selective dopamine D2 agonist. Neurochem Res 26:31–36. doi:10.1023/A:1007672414239
Iida M, Miyazaki I, Tanaka K, Kabuto H, Iwata-Ichikawa E, Ogawa N (1999) Dopamine D2 receptor-mediated antioxidant and neuroprotective effects of ropinirole, a dopamine agonist. Brain Res 838:51–59. doi:10.1016/S0006-8993(99)01688-1
Yoshioka M, Tanaka K, Miyazaki I, Fujita N, Higashi Y, Asanuma M et al (2002) The dopamine agonist cabergoline provides neuroprotection by activation of the glutathione system and scavenging free radicals. Neurosci Res 43:259–267. doi:10.1016/S0168-0102(02)00040-8
Le WD, Jankovic J, Xie W, Appel SH (2000) Antioxidant property of pramipexole independent of dopamine receptor activation in neuroprotection. J Neural Transm 107:1165–1173. doi:10.1007/s007020070030
Acknowledgments
This work was supported in part by Grants-in-Aid for Young Scientists (B) and for Scientific Research (C) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology, and by Health and Labour Sciences Research Grants for Research on Measures for Intractable Diseases, for Research on Regulatory Science of Pharmaceuticals and Medical Devices, and for Special Research from the Japanese Ministry of Health, Labour and Welfare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue article in honor of Dr. Akitane Mori.
Rights and permissions
About this article
Cite this article
Miyazaki, I., Asanuma, M. Approaches to Prevent Dopamine Quinone-Induced Neurotoxicity. Neurochem Res 34, 698–706 (2009). https://doi.org/10.1007/s11064-008-9843-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9843-1