Abstract
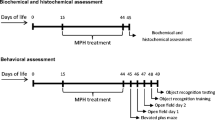
Methylphenidate (MPH) is frequently prescribed for the treatment of attention deficit/hyperactivity disorder. It was previously demonstrated that MPH altered brain metabolic activity. Most cell energy is obtained through oxidative phosphorylation, in the mitochondrial respiratory chain. However, there are still few studies about MPH effects on the brain of adult rats. Thus, in the present study we evaluated the effect of acute or chronic administration of MPH on the activities of mitochondrial respiratory chain complexes I–IV in the brain of adult rats. For acute administration, a single injection of MPH was given to 60-day-old rats. For chronic administration, MPH injections were given to 60-day-old rats once daily for 28 days. Our results showed that complexes I, II, III and IV were inhibited after acute or chronic MPH administration in the hippocampus, prefrontal cortex, striatum and cerebral cortex. On the other hand, cerebellum was not affected.


Similar content being viewed by others
References
Faraone SV, Sergeant J, Gillberg C et al (2003) The worldwide prevalence of ADHD: is it an American condition? World Psychiatry 2:104–113
Mannuzza S, Klein RG, Bessler A et al (1997) Educational and occupational outcome of hyperactive boys grown up. J Am Acad Child Adolesc Psychiatry 36:1222–1227
Mannuzza S, Klein RG, Bessle A et al (1998) Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry 155:493–498
Safer DJ, Allen RP (1989) Absence of tolerance to the behavioral effects of methylphenidate in hyperactive and inattentive children. J Pediat 115:1003–1008
Garland EJ (1998) Pharmacotherapy of adolescent attention deficit hyperactivity disorder: challenges, choices, caveats. J Psychopharmacol 12:385–395
Solanto MV (1998) Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res 94:127–152
Wigal T, Swanson JM, Regino R et al (1999) Stimulant medications for the treatment of ADHD: efficacy and limitations. Ment Retard Dev Disabil Res Rev 5:215–224
Biederman J, Mick E, Faraone SV (2000) Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry 157:816–818
Challman TD, Lipsky JJ (2000) Methylphenidate: its pharmacology and uses. Mayo Clin Proc 75:711–721
Kuczenski R, Segal DS (1997) Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem 68:2032–2037
Kuczenski R, Segal DS (2001) Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. J Pharmacol Exp Ther 296:876–883
Kuczenski R, Segal DS (2002) Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci 22:7264–7271
Greenhill LL (2001) Clinical effects of stimulant medication in attention-deficit/hyperactivity disorder (ADHD). In: Solanto MV, Arnsten AFT, Castellanos FX (eds) Stimulant drugs and ADHD: basic and clinical neuroscience. Oxford University Press, New York, pp 31–71
Teo SK, San RH, Wagner VO et al (2003) D-Methylphenidate is non-genotoxic in in vitro and in vivo assays. Mutat Res 537:67–79
Biederman J, Faraone SV (2005) Attention-deficit hyperactivity disorder. Lancet 366:237–248
Volkow ND, Wang G, Fowler JS et al (2001) Therapeutic doses of oral methylphenidate significantly increases extracellular dopamine in the human brain. J Neurosci 21:121
Volkow ND, Wang G, Fowler JS et al (2005) Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiatry 57:1410–1415
Wilens TE (2008) Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 28:S46–S53
Gray JD, Punsoni M, Tabori NE et al (2007) Methylphenidate administration to juvenile rats alters brain areas involved in cognition, motivated behaviors, appetite, and stress. J Neurosci 27:7196–7207
Valvassori SS, Frey BN, Martins MR et al (2007) Sensitization and cross-sensitization after chronic treatment with methylphenidate in adolescent Wistar rats. Behav Pharmacol 18:205–212
Chase TD, Brown RE, Carrey N et al (2003) Daily methylphenidate administration attenuates c-fos expression in the striatum of prepubertal rats. NeuroReport 14:769–772
Chase TD, Carrey N, Soo E et al (2007) Methylphenidate regulates activity regulated cytoskeletal associated but not brain-derived neurotrophic factor gene expression in the developing rat striatum. Neuroscience 144:969–984
Yano M, Steiner H (2005) Methylphenidate (Ritalin) induces Homer 1a and zif 268 expression in specific corticostriatal circuits. Neuroscience 132:855–865
LeBlanc-Duchin D, Taukulis HK (2009) Chronic oral methylphenidate induces post-treatment impairment in recognition and spatial memory in adult rats. Neurobiol Learn Mem 91:218–225
Robinson TE, Becker JB (1986) Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev 11:157–198
Segal DS, Kuczenski R (1994) Behavioral pharmacology of amphetamine. In: Cho AK, Segal DS (eds) Amphetamine and its analogues: psychopharmacology. Toxicology and Abuse Academic, San Diego, pp 115–150
Van der Schuren LJMJ, Kalivas PW (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology 151:99–120
Sax KW, Strakowski SM (1998) Enhanced behavioral response to repeated D-amphetamine and personality traits in humans. Biol Psychiatry 44:1192–1195
Strakowski SM, Sax KW (1998) Progressive behavioral response to repeated D-amphetamine challenge: further evidence for sensitization in humans. Biol Psychiatry 44:1171–1177
Strakowski SM, Sax KW, Rosenberg HL et al (2001) Human response to repeated low-dose D-amphetamine: evidence for behavioral enhancement and tolerance. Neuropsychopharmacology 25:548–554
Schenk S, Davidson E (1998) Stimulant pre-exposure sensitizes rats and humans to the rewarding effects of cocaine. NIDA Res Monogr 169:56–82
Laviola G, Adriani W, Terranova LM et al (1999) Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev 23:993–1010
Brandon CL, Marinelli M, Baker LK et al (2001) Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology 25:651–661
Porrino LJ, Lucignani G (1987) Different patterns of local brain energy metabolism associated with high and low doses of methylphenidate. Relevance to its action in hyperactive children. Biol Psychiatry 22:126–138
Scaini G, Fagundes AO, Rezin GT et al (2008) Methylphenidate increases creatine kinase activity in the brain of young and adult rats. Life Sci 83:795–800
Fagundes AO, Rezin GT, Zanette F et al (2007) Chronic administration of methylphenidate activates mitochondrial respiratory chain in brain of young rats. Int J Dev Neurosci 25:47–51
Gerasimov MR, Franceschi M, Volkow ND (2000) Synergistic interactions between nicotine and cocaine or methylphenidate depend on the dose of dopamine transporter inhibitor. Synapse 38:432–437
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–267.39
Cassina A, Radi R (1996) Differential inhibitory Aation of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys 328:309–316
Fischer JC, Ruitenbeek W, Berden JA et al (1985) Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta 153:23–26
Rustin P, Chretien D, Bourgeron T et al (1994) Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 228:35–51
Volkow ND, Wang GJ, Fowler JS et al (1998) Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry 155:1325–1331
Marsteller DA, Gerasimov MR, Schiffer WK et al (2002) Acute handling stress modulates methylphenidate-induced catecholamine overflow in the medial prefrontal cortex. Neuropsychopharmacology 27:163–170
LaVoie MJ, Hastings TG (1999) Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci 19:1484–1491
Page G, Peeters M, Najimi M et al (2001) Modulation of the neuronal dopamine transporter activity by the metabotropic glutamate receptor mGluR5 in rat striatal synaptosomes through phosphorylation mediated processes. J Neurochem 76:1282–1290
Spina MB, Cohen G (1989) Dopamine turnover and glutathione oxidation: implications for Parkinson disease. Proc Natl Acad Sci USA 86:1398–1400
Adam-Vizi V (2005) Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal 7:1140–1149
Navarro A, Boveris A (2007) The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol 292:670–686
Gruno M, Peet N, Tein A et al (2008) Atrophic gastritis: deficient complex I of the respiratory chain in the mitochondria of corpus mucosal cells. J Gastroenterol 43:780–788
Sen T, Sen N, Jana S et al (2007) Depolarization and cardiolipin depletion in aged rat brain mitochondria: relationship with oxidative stress and electron transport chain activity. Neurochem Int 50:719–725
Massicotte C, Knight K, Van der Schyf CJ et al (2005) Effects of organophosphorus compounds on ATP production, mitochondrial integrity in cultured cells. Neurotox Res 7(3):203–217
Barja G, Herrero A (1998) Localization at complex I and mechanism of the higher free radical production of brain nonsynaptic mitochondria in the short-lived rat than in the longevous pigeon. J Bioenerg Biomembr 30:235–243
Gassner B, Wuthrich A, Scholtysik G et al (1997) The pyrethroids permethrin and cyhalothrin are potent inhibitors of the mitochondrial complex I. J Pharmacol Exp Ther 281:855–860
Sherer TB, Betarbet R, Kim JH et al (2003) Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol 179:9–16
Beal MF (1992) Does impairment of energy metabolism result in excitotoxic neuronal death in neurological illnesses? Ann Neurol 31:119–130
Heales SJ, Bolaños JP, Stewart VC (1999) Nitric oxide, mitochondria and neurological disease. Biochim Biophys Acta 1410:215–228
Blass JP (2001) Brain metabolism and brain disease: is metabolic deficiency the proximate cause of Alzheimer dementia? J Neurosci Res 66:851–856
Schurr A (2002) Energy metabolism, stress hormones and neural recovery from cerebral ischemia/hypoxia. Neurochem Int 41:1–8
Land JM, Morgan-Hughes JA, Hargreaves I et al (2004) Mitochondrial disease: a historical, biochemical, and London perspective. Neurochem Res 29:483–491
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795
Andreazza AC, Frey BN, Valvassori SS et al (2007) DNA damage in rats after treatment with methylphenidate. Prog Neuropsychopharmacol Biol Psychiatry 31:1282–1288
Andreu AL, Arbos MA, Perez-Martos A et al (1998) Reduced mitochondrial DNA transcription in senescent rat heart. Biochem Biophys Res Commun 252:577–581
Acknowledgments
This work was supported by grants from Conselho Nacional de Pesquisa e Desenvolvimento (CNPq) and Universidade do Extremo Sul Catarinense (UNESC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fagundes, A.O., Scaini, G., Santos, P.M. et al. Inhibition of Mitochondrial Respiratory Chain in the Brain of Adult Rats After Acute and Chronic Administration of Methylphenidate. Neurochem Res 35, 405–411 (2010). https://doi.org/10.1007/s11064-009-0069-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-009-0069-7