Abstract
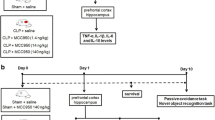
Sepsis is characterized by biochemical alterations in the central nervous system at early times and cognitive impairment at late times after induction in sepsis animal model. In order to understand at least in part the mechanism of disease, we have evaluated the effects of sepsis on cytokine levels in the cerebrospinal fluid (CSF); oxidative parameters; the activity of the electron transport chain enzymes; and creatine kinase (CK) activity in the brain of sepsis survivor rats 10 days after cecal ligation and perforation (CLP). Male Wistar rats underwent CLP with “basic support” or sham-operated. Ten days after surgery, the animals were killed and prefrontal cortex, cortex, hippocampus, striatum, cerebellum, and CSF were obtained. It was found a decrease in the levels of TNF-α (P = 0.001), IL-1β (P = 0.008), IL-6 (P = 0.038), and IL-10 (P = 0.022) in the CSF; an increase in the TBARS only hippocampus (0.027); an up-regulation in the activity of complex II (P = 0.024), III (P = 0.018), and IV (P = 0.047) only in the prefrontal cortex; a decrease in the CK activity in the cerebellum (P = 0.001) and striatum (P = 0.0001), and an increase in the hippocampus (P = 0.0001) and cortex (P = 0.0001). Oxidative stress and mitochondrial alterations observed during early times in sepsis, persisted up to 10 days after surgery. The cytokines levels during the early times were found at high levels, decreasing to low levels after 10 days. In conclusion, these findings may contribute for a better comprehension of the cognitive damage in sepsis survivor rats.




Similar content being viewed by others
References
Angus DC, Linde-Zwirble WT, Lidicker J et al (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310
Marshall JC, Deitch C, Moldawer LL, Opal S, Redl H, Van der Poll T (2005) Preclinical models of shock and sepsis: what can their tells us? Shock 24:1Y6
Comim CM, Constantino LC, Barichello T et al (2009) Cognitive impairment in the septic brain. Curr Neurovasc Res 6:194–203
Rietschel ET, Brade H, Holst O et al (1996) Bacterial endotoxin: chemical constitution, biological recognition, host response, and immunological detoxification. Curr Top Microbiol Immunol 216:39–81
Wilson JX, Young GB (2003) Progress in clinical neurosciences: sepsis- associated encephalopathy: evolving concepts. Can J Neuroll Sci 30:98–105
Chao CC, Hu S, Peterson PK (1995) Glia, cytokines, and neurotoxicity. Crit Rev Neurobiol 9:189–205
Hu S, Peterson PK, Chao CC (1997) Cytokine-mediated neuronal apoptosis. Neurochem Int 30:427–431
Messaris E, Memos N, Chatzigianni E et al (2004) Time-dependent mitochondrial-mediated programmed neuronal cell death prolongs survival in sepsis. Crit Care Med 32:1764–1770
Semmler A, Okulla T, Sastre M et al (2005) Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat 30:144–157
Barichello T, Fortunato JJ, Vitali AM et al (2006) Oxidative variables in the rat brain after sepsis induced by cecal ligation and perforation. Crit Care Med 34:886–889
Abd El-Gawad HM, Khalifa AE (2001) Quercetin, coenzyme Q10, and Lcanavanine as protective agents against lipid peroxidation and nitric oxide generation in endotoxin-induced shock in rat brain. Pharmacol Res 43:257–263
Boczkowski J, Lisdero CL, Lanone S (1999) Endogenous peroxynitrite mediates mitochondrial dysfunction in rat diaphragm during endotoxemia. Faseb J 13:1637–1646
Comim CM, Rezin GT, Scaini G et al (2008) Mitochondrial respiratory chain and creatine kinase activities in rat brain after sepsis induced by cecal ligation and perforation. Mitochondrion 8:313–318
Brealey D, Brand M, Hargreaves I et al (2002) Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360:219–223
Protti A, Singer M (2006) Bench-to-bedside review: potential strategies to protect or reverse mitochondrial dysfunction in sepsis-induced organ failure. Crit Care 10:228
Crouser ED (2004) Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion 4:729–741
Crouser ED, Julian MW, Blaho DV et al (2002) Endotoxin-induced mitochondrial damage correlates with impaired respiratory activity. Crit Care Med 30:276–284
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795
Crouser ED, Julian MW, Dorinsky PM (1999) Ileal VO2-DO2 alterations induced by endotoxin correlate with severity of mitochondrial injury. Am J Respir Crit Care Med 160:1347–1353
Tuon L, Comim CM, Petronilho F et al (2008) Time-dependent behavioral recovery after sepsis in rats. Intensive Care Med 34:1724–1731
Ritter C, Andrades M, Frota Júnior ML et al (2003) Oxidative parameters and mortality in sepsis induced by cecal ligation and perforation. Intensive Care Med 29:1782–1789
Papadopoulos MC, Davies DC, Moss RF et al (2000) Pathophysiology of septic encephalopathy: a review. Crit Care Med 28:3019–3024
Rothwell NJ, Luheshi GN (2000) nterleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci 23:618–625
van den Berghe G, Wouters P, Weekers F et al (2001) Intensive insulin therapy in critically ill patients. N Engl J Med 345:1359–1367
Aly H, Khashaba MT, El-Ayouty M et al (2006) IL-1beta, IL-6 and TNF-alpha and outcomes of neonatal hypoxic ischemic encephalopathy. Brain Dev 28:178–182
Oberholzer A, Oberholzer C, Moldawer LL (2001) Sepsis syndromes: understanding the role of innate and acquired immunity. Shock 16:83–96
Ertel W, Kremer JP, Kenney J et al (1995) Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood 85:1341–1347
Dal-Pizzol F, Ritter C, Cassol-Jr OJ et al (2010) Oxidative mechanisms of brain dysfunction during sepsis. Neurochem Res 35:1–12
Munford RS, Pugin J (2001) Normal response to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med 163:316–321
Zhang X, Morrison DC (1993) Lipopolysaccharide structure-function relationship in activation versus reprogramming of mouse peritoneal macrophages. J Leukoc Biol 54:444–450
Batandier C, Guigas B, Detaille D et al (2006) The in reactive oxygen species production induced by a reverse-electron flux at respiratory chain complex 1 is hampered by metformin. J Bioenerg Biomembr 38:33–42
Adibhatla RM, Hatcher JF (2008) Altered lipid metabolism in brain injury and disorders. Subcell Biochem 49:241–268
Wenk MR (2005) The emerging field of lipidomics. Nat Rev Drug Discov 4:594–610
Sastry PS (1985) Lipids of nervous tissue: composition and metabolism. Prog Lipid Res 24:69–176
Dringen R (2000) Metabolism and functions of glutathione in brain. Prog Neurobiol 62:649–671
Crouser ED, Julian MW, Huff JE et al (2004) Abnormal permeability of inner and outer mitochondrial membranes contributes independently to mitochondrial dysfunction in the liver during acute endotoxemia. Crit Care Med 32:478–488
Enns GM (2003) The contribution of mitochondria to common disorders. Mol Genet Metab 80:11–26
Navarro A, Boveris A (2007) The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol 292:C670–C686
Whittingham TS, Lipton P (1981) Cerebral synaptic transmission during anoxia is protected by creatine. J Neurochem 37:1618–1621
Stachowiak O, Dolder M, Wallimann T et al (1998) Mitochondrial creatine kinase is a prime target of peroxynitrite-induced modification and inactivation. J Biol Chem 273:16694–16699
Acknowledgments
This research was supported by grants from CNPq (ELS, JQ and FD-P), and UNESC (ELS, JQ and FD-P). ELS, JQ and FD-P are CNPq Research Fellows. CMC is holder of a CNPq Studentship, LSC is holder of a FAPESC studentship and GTR, GS and FP are holders of CAPES studentships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Comim, C.M., Cassol-Jr, O.J., Constantino, L.S. et al. Alterations in Inflammatory Mediators, Oxidative Stress Parameters and Energetic Metabolism in the Brain of Sepsis Survivor Rats. Neurochem Res 36, 304–311 (2011). https://doi.org/10.1007/s11064-010-0320-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0320-2