Abstract
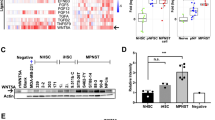
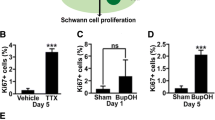
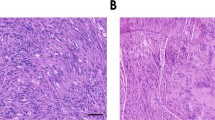
Malignant peripheral nerve sheath (MPNST) cell lines derived from patients with neurofibromatosis type 1 (NF!) were found to have basal cAMP levels which are two-fold higher than cAMP levels in normal human adult Schwann cells (nHSC). PCR analysis also revealed that normal adult human Schwann cells express mRNA for types Ill, IV, and IX adenylyl cyclase (AC) while NF1 MPNST cells express AC mRNA of types II, V, and VIII in addition to expressing all the isoforms of normal adult human Schwann cells. Further PCR analysis revealed that NF1 MPNST lines express mRNA for EP2 and EP4 prostaglandin receptors whereas nHSC only express mRNA for the EP2 receptor. Exogenous prostaglandins alone or in combination with PDGF BB induced greater increases in cAMP levels and proliferation of NF1 MPNST cells compared to nHSC. We conclude that aberrant cAMP signaling in NF1 MPNST cells contributes to tumor formation in NF1 patients.






Similar content being viewed by others
References
Friedman JM, Gutmann DH, MacCollin M, Ricardi VM (1999) Neurofibromatosis: phenotype, natural history, and pathogenesis, 3rd edn. The Johns Hopkins University Press, Baltimore
Rosenbaum T, Petrie KM, Ratner N (1997) Neurofibromatosis type 1: genetic and cellular mechanisms of peripheral nerve tumor formation. Neurosci 3:412–420
Morioka N, Tsuchida T, Etoh T, Ishibashi Y, Otsuka F (1990) A case of neurofibrosarcoma associated with neurofibromatosis: light microscopic, ultrastructural, immunohistochemical and biochemical investigations. J Dermatol 17:312–316
Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A (2002) Malignant peripheral nerve sheath tumors in neurofibromatosis 1. J Med Genet 39:311–314
Sangueza OP, Requena L (1998) Neoplasms with neural differentiation: a review. Part II. Malignant neoplasms. Am J Dermapathol 20:89–102
Ducatman BS, Scheithauer BW, Piepgrass DG, Reiman HM, Ilstrup DM (1986) Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer 57:2006–2021
Wong WW, Hirose T, Scheithauer BW, Schild SE, Gunderson LL (1998) Malignant peripheral nerve sheath tumor: analysis of treatment outcome. Int J Radiat Oncol Phys 42:351–360
Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J (1992) Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature 356:713–715
DeClue JE, Papageorge A, Diehl SR, Ratner N, Vass WC, Lowy DR (1992) Abnormal regulation of mammalian p21 ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell 60:265–273
Feldkamp MM, Angelov L, Guha A (1999) Neurofibromatosis type 1 peripheral nerve tumors: aberrant activation of the ras pathway. Surg Neurol 1:211–218
Thomas SL, Deadwyler GD, Tang J, Stubbs EB Jr, Muir D, Hiatt KK, Clapp DW, De Vries GH (2006) Reconstitution of the NF1 GAP-related domain in NF1-deficient human Schwann cells. Biochem Biophys Res Commun 348:971–980
Li Y, Rao PK, Wen R, Song Y, Muir D, Wallace P, van Home SJ, Tennekoon GI, Kadesch T (2004) Notch and Schwann cell transformation. Oncogene 23:1146–1152
Kim HA, Ratner N, Roberts TM, Stiles C (2001) Schwann cell proliferative responses to cAMP and Nf1 are mediated by cyclin Dl. J Neurosci 21:1110–1116
Tong J, Hannan F, Zhu Y, Bemards A, Zhong Y (2002) Neurofibromin regulates G protein-stimulated adenylyl cyclase activity. Nat Neurosci 5:95–96
Izawa I, Tamaki N, Saya H (1996) Phosphorylation of neurofibromatosis type 1 gene product (neurofibromin) by cAMP-dependent protein kinase. FEBS Lett 382(1–2):53–59
The I, Hannigan GE, Cowley GS, Reginald S, Zhong Y, Gusella JF, Hariharan IK, Bernards A (1997) Rescue of a Drosophila NF1 mutant phenotype by protein kinase A. Science 276(5313):791–794
Deadwyler GD, Dang I, Nelson J, Srikanth M, DeVries GH (2004) Prostaglandin E2 metabolism is activated in Schwann cell lines derived from human NF1 malignant peripheral nerve sheath tumors. Neuron Glia Biol 1(2):149–155
Wang D, Dubois RN (2010) Eicosanoids and cancer. Nat Rev Cancer 10(3):181–193
Ghosh N, Chaki R, Mandal V, Mandal SC (2010) COX-2 as a target for cancer chemotherapy. Pharmacol Rep 62(2):233–244
Prasad KN, Cole WC, Yan XD, Nahreini P, Kumar B, Hanson A, Prasad JE (2003) Defects in cAMP-pathway may initiate carcinogenesis in dividing nerve cells: a review. Apoptosis 8(6):579–586
Badache A, DeVries GH (1998) Neurofibrosarcoma-derived Schwann cells overexpress platelet-derived growth factor (PDGF) receptors and are induced to proliferate by PDGF BB. J Cell Phys 177:334–342
DeVries GH, Boullerne AI (2010) Glial cell lines: an overview. Neurochem Res 35:1978–2000
Casella GT, Wieser R, Bunge RP, Margitich IS, Katz J, Olson L, Wood PM (2000) Density dependent regulation of human Schwann cell proliferation. Glia 30:165–177
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162(1):156–159
Clive DR, Lopez TJ, DeVries GH (1998) Quantitation in PO mRNA by polymerase chain reaction in primary culture Schwann cells stimulated by axolemma-enriched fraction. J Neurosci Methods 81:24–34
Weinmaster G, Lemke G (1990) Cell-specific cyclic AMP-mediated induction of PDGF receptor. EMBO J 9:915–920
Menghi F, Jacques TS, Barenco M, Schwalbe EC, Clifford SC, Hubank M, Ham J (2011) Genome-wide analysis of alternative splicing in medulloblastoma identifies splicing patterns characteristic of normal cerebellar development. Cancer Res [Epub ahead of print]
Coleman RA, Smith WL, Narumiya S (1994) International union of pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev 46:205–229
Sobue G, Shuman S, Pleasure D (1986) Schwann cell responses to cyclic AMP: proliferation, changes in shape, and appearance of surface galactocerebroside. Brain Res 362:23–32
Walikonis RS, Poduslo JF (1998) Activity of cyclic AMP phosphodiesterases and adenylyl cyclase in peripheral nerve after crush and permanent transection injuries. J Biol Chem 273:9070–9077
Fieber LA (1998) Ionic currents in normal and neurofibromatosis type 1-affected human Schwann cells: induction of tumor cell K current in normal Schwann cells by cyclic AMP. J Neurosci Res 54(4):495–506
Warrington NM, Gianino SM, Jackson E, Goldhoff P, Garbow JR, Piwnica-Worms D, Gutmann DH, Rubin JB (2010) Cyclic AMP suppression is sufficient to induce gliomagenesis in a mouse model of neurofibromatosis-1. Cancer Res 70(14):5717–5727
Hegedus B, Dasgupta B, Shin JE, Emnett RJ, Hart-Mahon EK, Elghazi L, Bernal-Mizrachi E, Gutman DH (2007) Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP-and Ras-dependent mechanisms. Cell Stem Cell 1(4):443457
Kim AK, DeClue JE, Ratner N (1997) cAMP-dependent protein kinase A is required for Schwann cell growth: interactions between the cAMP and neuregulin/tyrosine kinase pathways. J Neurosci Res 49:236–247
Marshall JS, Gomi K, Blennerhassett M, Bienenstock J (1999) Nerve growth factor modifies the expression of inflammatory cytokines by mast cells via a prostanoid-dependent mechanism. J Immunol 162:4271–4276
Isaccson P (1976) Mast cells in benign nerve sheath tumours. J Pathol 119:193–196
Ricardi VM (1992) Neurofibromatosis. Phenotype, natural history of pathogenesis, 2nd edn. The John Hopkins University Press, Baltimore
Mrabet-Dahbi S, Metz M, Dudeck A, Zuberbier T, Maurer M (2009) Murine mast cells secrete a unique profile of cytokines and prostaglandins in response to distinct TLR2 ligands. Exp Dermatol 18(5):437–444
Constable AL, Armati PJ, Toyka KV, Hartung HP (1994) Production of prostanoids by Lewis rat Schwann cells in vitro. Brain Res 635:75–80
Feng L, Yunoue S, Tokuo H, Zhang D, Patrakitkomjorn S, Ichimura T, Saya H, Araki N (2004) PKA phosphorylation and 14–3–3 interaction regulate the function of neurofibromatosis type 1 tumor suppressor, neurofibomin. FEBS Lett 557(1–3):275–282
Acknowledgments
This research was supported by US Army Medical Research and Material Command; (Contact grant number: DAMD17-98-8607). Salary support for G.H.D.V. was provided by a Career Research Health Science Award from the Department of Veterans Affairs. The authors thank Julie Litz for secretarial assistance. We are honored to make this contribution in tribute to the career of Robert K. Yu who we have been known for many years. Our association began over 40 years ago in the Saul Korey Department of Neurology at the Albert Einstein College of Medicine where we both carried out very enjoyable and productive postdoctoral studies. Bob also served as my Chairman for 7 years at the Medical College of Virginia. He is well known in neurochemistry for his pioneering work in the field of glycosphingolipids. His encouragement and hospitality have been a positive factor to myself and many others with whom he interacted over the years. His warm personality and helpful insights have been also been a blessing to many. On a more personal note he has been a gentleman and true friend to me and many others over the years. We wish him continued success and health in his continuing quest to understand the biological function of the complex glycosphingolipids which he continues to investigate.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: In Honor of Dr. Robert Yu.
Rights and permissions
About this article
Cite this article
Dang, I., De Vries, G.H. Aberrant cAMP Metabolism in NF1 Malignant Peripheral Nerve Sheath Tumor Cells. Neurochem Res 36, 1697–1705 (2011). https://doi.org/10.1007/s11064-011-0433-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0433-2