Abstract
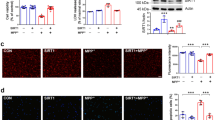
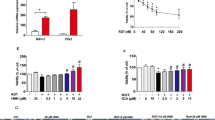
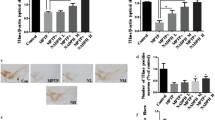
Parkinson’s disease (PD) is one of the most common neurodegenerative diseases, which is characterized by progressive degeneration of nigrostriatal dopaminergic neurons. There is a growing consensus that mitochondrial dysfunction and oxidative stress play a crucial role in PD pathogenesis. Sirtuin3 (SIRT3) is the major mitochondria NAD+-dependent deacetylase that acts as a regulator of mitochondrial protein function; it is essential for maintaining mitochondrial integrity. Although SIRT3 was reported to have anti-oxidative stress activity in an in vitro study, there is no explicit in vivo evidence for the involvement of SIRT3 in the etiology of PD. The present study shows that SIRT3 null mice do not exhibit motor and non-motor deficits compared with wild-type controls. However, SIRT3 deficiency dramatically exacerbated the degeneration of nigrostriatal dopaminergic neurons in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mice. SIRT3 null mice exposed to MPTP also exhibited decreased superoxide dismutase 2, a specific mitochondrial antioxidant enzyme, and reduced glutathione peroxidase expression compared with wild-type controls. Taken together, these findings strongly support that SIRT3 has a possible role in MPTP-induced neurodegeneration via preserving free radical scavenging capacity in mitochondria.





Similar content being viewed by others
References
Moore DJ, West AB, Dawson VL, Dawson TM (2005) Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci 28:57–87
Lees AJ, Hardy J, Revesz T (2009) Parkinson’s disease. Lancet 373(9680):2055–2066
Perier C, Vila M (2012) Mitochondrial biology and Parkinson’s disease. Cold Spring Harb Perspect Med 2(2):a009332
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7113):787–795
Orrenius S, Gogvadze V, Zhivotovsky B (2007) Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol 47:143–183
Donmez G, Guarente L (2010) Aging and disease: connections to sirtuins. Aging Cell 9(2):285–290
Haigis MC, Sinclair DA (2010) Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol 5:253–295
Bell EL, Guarente L (2011) The SirT3 divining rod points to oxidative stress. Mol Cell 42(5):561–568
Ahn BH, Kim HS, Song S, Lee IH, Liu J et al (2008) A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA 105:14447–14452
Qiu X, Brown K, Hirschey MD, Verdin E, Chen D (2010) Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12:662–667
Someya S, Yu W, Hallows WC, Xu J, Vann JM et al (2010) Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143:802–812
Tao R, Coleman MC, Pennington JD, Ozden O, Park SH et al (2010) Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell 40:893–904
Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y et al (2009) Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging 1(9):771–783
Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B et al (2010) SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464(7285):121–125
Jackson-Lewis V, Przedborski S (2007) Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc 2(1):141–151
Chiba K, Peterson LA, Castagnoli KP, Trevor AJ, Castagnoli N Jr (1985) Studies on the molecular mechanism of bioactivation of the selective nigrostriatal toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Drug Metab Dispos 13(3):342–347
Cleeter MW, Cooper JM, Schapira AH (1992) Irreversible inhibition of mitochondrial complex I by 1-methyl-4-phenylpyridinium: evidence for free radical involvement. J Neurochem 58(2):786–789
Hantraye P, Brouillet E, Ferrante R, Palfi S, Dolan R et al (1996) Inhibition of neuronal nitric oxide synthase prevents MPTP-induced parkinsonism in baboons. Nat Med 2(9):1017–1021
Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS et al (2007) Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 27(24):8807–8814
Cryan JF, Holmes A (2005) The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 4(9):775–790
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85(3):367–370
Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G et al (2001) The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem 76(5):1265–1274
Nagatsu T, Levitt M, Udenfriend S (1964) Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis. J Biol Chem 239:2910–2917
Flynn JM, Melov S (2013) SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic Biol Med 62:4–12
Verdin E, Hirschey MD, Finley LW, Haigis MC (2010) Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci 35(12):669–675
Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N et al (2010) SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell 17(1):41–52
Piao Y, Kim HG, Oh MS, Pak YK (2012) Overexpression of TFAM, NRF-1 and myr-AKT protects the MPP(+)-induced mitochondrial dysfunctions in neuronal cells. Biochim Biophys Acta 1820(5):577–585
Zhang X, Zhou JY, Chin MH, Schepmoes AA, Petyuk VA et al (2010) Region-specific protein abundance changes in the brain of MPTP-induced Parkinson’s disease mouse model. J Proteome Res 9(3):1496–1509
Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA et al (1999) Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol 46(4):598–605
Sedelis M, Hofele K, Auburger GW, Morgan S, Huston JP et al (2000) MPTP susceptibility in the mouse: behavioral, neurochemical, and histological analysis of gender and strain differences. Behav Genet 30(3):171–182
Balaban RS, Nemoto S, Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120(4):483–495
Piantadosi CA, Suliman HB (2012) Redox regulation of mitochondrial biogenesis. Free Radic Biol Med 53(11):2043–2053
Ghosh N, Ghosh R, Mandal SC (2011) Antioxidant protection: a promising therapeutic intervention in neurodegenerative disease. Free Radic Res 45(8):888–905
Acknowledgments
We would like to thank Dr. Shui-Ying Ng and CNR Imaging Core for excellent technical assistance on microscope equipment, Dr. Jennifer Newman and CNR Behavior Core for instruction on behavior tests, and Dr.Yongjie Yang for the use of his laboratory’s freezing microtome equipment. Also we express thanks to Prof. Rob Jackson for his critical reading of the manuscript. This study was supported by the Tufts University Department of Neuroscience startup fund (to G.D.) and the Tufts Center for Neuroscience Research Pilot Award (to G.D.).
Conflict of interest
The authors declare there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, L., Peritore, C., Ginsberg, J. et al. SIRT3 Attenuates MPTP-Induced Nigrostriatal Degeneration Via Enhancing Mitochondrial Antioxidant Capacity. Neurochem Res 40, 600–608 (2015). https://doi.org/10.1007/s11064-014-1507-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1507-8