Abstract
Purpose
This study examines the anti-cancer effect of carnosol in human prostate cancer PC3 cells and its role in modulating multiple signaling pathways associated with carcinogenesis.
Methods
PC3 cells were treated with carnosol and were evaluated using a flow cytometry, a protein array and Western blot analysis to identify signaling pathways targeted by carnosol.
Results
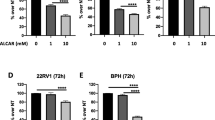
Using an MTT assay we found that carnosol (10–70 μM) decreases cell viability in a time and dose-dependent manner. Further analysis using flow cytometry as well as biochemical analysis identified G2-phase cell cycle arrest. To establish a more precise mechanism, we performed a protein array that evaluated 638 proteins involved in cell signaling pathways. The protein array identified 5′-AMP-activated protein kinase (AMPK), a serine/threonine protein kinase involved in the regulation of cellular energy balance as a potential target. Further downstream effects consistent with cancer inhibition included the modulation of the mTOR/HSP70S6k/4E-BP1 pathway. Additionally, we found that carnosol targeted the PI3K/Akt pathway in a dose dependent manner.
Conclusions
These results suggest that carnosol targets multiple signaling pathways that include the AMPK pathway. The ability of carnosol to inhibit prostate cancer in vitro suggests carnosol may be a novel agent for the management of PCa.








Similar content being viewed by others
Abbreviations
- AICAR:
-
5-aminoimidazole-4-carboxamide ribonucleoside
- AMPK:
-
5′-AMP-activated protein kinase
- cdks:
-
cyclin dependent kinases
- DMSO:
-
dimethyl sulfoxide
- FBS:
-
fetal bovine serum
- LH-RH:
-
luteinizing hormone-releasing hormone
- mTOR:
-
mammalian target of rapamycin
- PBS:
-
phophsphate buffered saline
- Pca:
-
prostate cancer
- PI3K:
-
phosphatidylinositol 3-kinase
- PTEN:
-
phosphatase and tensin homologue deleted on chromosome ten
- TSC2:
-
tuberous sclerosis complex 2
References
A. Jemal, R. Siegel, E. Ward, T. Murray, J. Xu, and M. J. Thun. Cancer statistics. CA Cancer J. Clin. 57:43–66 (2007).
D. N. Syed, N. Khan, F. Afaq, and H. Mukhtar. Chemoprevention of prostate cancer through dietary agents: progress and promise. Cancer Epidemiol. Biomarkers Prev. 16:2193–2203 (2007).
S. R. Denmeade, X. S. Lin, and J. T. Isaacs. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate 28:251–265 (1996).
J. J. Johnson, and H. Mukhtar. Curcumin for chemoprevention of colon cancer. Cancer Lett. 255:170–181 (2007).
T. Kakizoe. Chemoprevention of cancer—focusing on clinical trials. Jpn. J. Clin. Oncol. 33:421–442 (2003).
O. I. Aruoma, J. P. Spencer, R. Rossi, R. Aeschbach, A. Khan, N. Mahmood, A. Munoz, A. Murcia, J. Butler, and B. Halliwell. An evaluation of the antioxidant and antiviral action of extracts of rosemary and Provencal herbs. Food Chem. Toxicol. 34:449–456 (1996).
M. T. Huang, C. T. Ho, Z. Y. Wang, T. Ferraro, Y. R. Lou, K. Stauber, W. Ma, C. Georgiadis, J. D. Laskin, and A. H. Conney. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 54:701–708 (1994).
O. I. Aruoma, B. Halliwell, R. Aeschbach, and J. Loligers. Antioxidant and pro-oxidant properties of active rosemary constituents: carnosol and carnosic acid. Xenobiotica 22:257–268 (1992).
K. Singletary, C. MacDonald, and M. Wallig. Inhibition by rosemary and carnosol of 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett. 104:43–48 (1996).
A. E. Moran, A. M. Carothers, M. J. Weyant, M. Redston, and M. M. Bertagnolli. Carnosol inhibits beta-catenin tyrosine phosphorylation and prevents adenoma formation in the C57BL/6J/Min/+ (Min/+) mouse. Cancer Res. 65:1097–1104 (2005).
J. M. Visanji, D. G. Thompson, and P. J. Padfield. Induction of G2/M phase cell cycle arrest by carnosol and carnosic acid is associated with alteration of cyclin A and cyclin B1 levels. Cancer Lett. 237:130–136 (2006).
H. Motoshima, B. J. Goldstein, M. Igata, and E. Araki. AMPK and cell proliferation—AMPK as a therapeutic target for atherosclerosis and cancer. J. Physiol. 574:63–71 (2006).
X. Xiang, A. K. Saha, R. Wen, N. B. Ruderman, and Z. Luo. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem. Biophys. Res. Commun. 321:161–167 (2004).
D. G. Hardieand, and D. Carling. The AMP-activated protein kinase-fuel gauge of the mammalian cell? Eur. J. Biochem. 246:259–273 (1997).
B. E. Kemp, D. Stapleton, D. J. Campbell, Z. P. Chen, S. Murthy, M. Walter, A. Gupta, J. J. Adams, F. Katsis, B. van Denderen, I. G. Jennings, T. Iseli, B. J. Michell, and L. A. Witters. AMP-activated protein kinase, super metabolic regulator. Biochem. Soc. Trans. 31:162–168 (2003).
Y. Fang, M. Vilella-Bach, R. Bachmann, A. Flanigan, and J. Chen. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 294:1942–1945 (2001).
T. J. Guan, F. J. Qin, J. H. Du, L. Geng, Y. Y. Zhang, and M. Li. AICAR inhibits proliferation and induced S-phase arrest, and promotes apoptosis in CaSki cells. Acta. Pharmacol. Sin. 28:1984–1990 (2007).
D. R. Bolster, S. J. Crozier, S. R. Kimball, and L. S. Jefferson. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J. Biol. Chem. 277:23977–23980 (2002).
K. Imamura, T. Ogura, A. Kishimoto, M. Kaminishi, and H. Esumi. Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole- 4-carboxamide-1-beta-d-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem. Biophys. Res. Commun. 287:562–567 (2001).
N. Khan, F. Afaq, M. H. Kweon, K. Kim, and H. Mukhtar. Oral consumption of pomegranate fruit extract inhibits growth and progression of primary lung tumors in mice. Cancer Res. 67:3475–3482 (2007).
D. Xiao, and S. V. Singh. Diallyl trisulfide, a constituent of processed garlic, inactivates Akt to trigger mitochondrial translocation of BAD and caspase-mediated apoptosis in human prostate cancer cells. Carcinogenesis. 27:533–540 (2006).
B. T. Nave, M. Ouwens, D. J. Withers, D. R. Alessi, and P. R. Shepherd. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem. J. 344(Pt 2):427–431 (1999).
K. Inoki, T. Zhu, and K.L. Guan. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 115:577–590 (2003).
Acknowledgements
This work was supported by a Clinical and Translational Science Award (CTSA) training grant (J.Johnson) through the Institute for Clinical and Translation Research (ICTR) at the University of Wisconsin. (NIH 1KL2RR025012-01 and K12 RR023268).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johnson, J.J., Syed, D.N., Heren, C.R. et al. Carnosol, a Dietary Diterpene, Displays Growth Inhibitory Effects in Human Prostate Cancer PC3 Cells Leading to G2-Phase Cell Cycle Arrest and Targets the 5′-AMP-Activated Protein Kinase (AMPK) Pathway. Pharm Res 25, 2125–2134 (2008). https://doi.org/10.1007/s11095-008-9552-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9552-0