ABSTRACT
Purpose
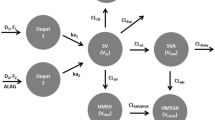
To develop a population physiologically-based pharmacokinetic (PBPK) model for simvastatin (SV) and its active metabolite, simvastatin acid (SVA), that allows extrapolation and prediction of their concentration profiles in liver (efficacy) and muscle (toxicity).
Methods
SV/SVA plasma concentrations (34 healthy volunteers) were simultaneously analysed with NONMEM 7.2. The implemented mechanistic model has a complex compartmental structure allowing inter-conversion between SV and SVA in different tissues. Prior information for model parameters was extracted from different sources to construct appropriate prior distributions that support parameter estimation. The model was employed to provide predictions regarding the effects of a range of clinically important conditions on the SV and SVA disposition.
Results
The developed model offered a very good description of the available plasma SV/SVA data. It was also able to describe previously observed effects of an OATP1B1 polymorphism (c.521 T > C) and a range of drug-drug interactions (CYP inhibition) on SV/SVA plasma concentrations. The predicted SV/SVA liver and muscle tissue concentrations were in agreement with the clinically observed efficacy and toxicity outcomes of the investigated conditions.
Conclusions
A mechanistically sound SV/SVA population model with clinical applications (e.g., assessment of drug-drug interaction and myopathy risk) was developed, illustrating the advantages of an integrated population PBPK approach.






Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the concentration-time curve
- CLR:
-
Clarithromycicn
- Cmax:
-
Maximum concentration
- CYP:
-
Cytochrome P450
- DDIs:
-
Drug-drug interactions
- DTZ:
-
Diltiazem
- ERY:
-
Erythromycin
- F:
-
Oral bioavailability
- Fa :
-
Fraction absorbed into gut wall
- Fg :
-
Fraction reaching gut wall that escapes intestinal first-pass metabolism
- Fh :
-
Fraction reaching liver that escapes hepatic first-pass metabolism
- FREC :
-
Parameter that quantifies the magnitude of the recycling (inter-conversion) process
- FOCE-I:
-
First order conditional estimation method with interaction
- HMG-CoA:
-
3-hydroxy-3-methylglutaryl-coenzyme A
- IMPMAP:
-
Monte-Carlo importance sampling assisted by mode a posteriori estimation
- ITZ:
-
Itraconazole
- IVIVE:
-
In vitro - in vivo extrapolation
- IW:
-
Inverse-Wishart distribution
- LDL:
-
Low-density lipoprotein
- LOQ:
-
Limit of quantification
- MAP:
-
Maximum a posteriori
- MCMC:
-
Markov chain Monte Carlo
- OATP:
-
Organic anion transporting polypeptide
- PBPK:
-
Physiologically-based pharmacokinetic
- PK/PD:
-
Pharmacokinetic/pharmacodynamic
- RSE:
-
Relative standard error
- SNP:
-
Single nucleotide polymorphism
- SV:
-
Simvastatin (lactone form)
- SVA:
-
Simvastatin acid (acid form)
- VPC:
-
Visual predictive check
References
Mauro VF. Clinical pharmacokinetics and practical applications of simvastatin. Clin Pharmacokinet. 1993;24(3):195–202.
Collins R, Armitage J, Parish S, Sleight P, Peto R. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22.
Vickers S, Duncan CA, Chen IW, Rosegay A, Duggan DE. Metabolic disposition studies on simvastatin, a cholesterol-lowering prodrug. Drug Metab Dispos. 1990;18(2):138–45.
Prueksaritanont T, Qiu Y, Mu L, Michel K, Brunner J, Richards KM, et al. Interconversion pharmacokinetics of simvastatin and its hydroxy acid in dogs: effects of gemfibrozil. Pharm Res. 2005;22(7):1101–9.
Prueksaritanont T, Subramanian R, Fang X, Ma B, Qiu Y, Lin JH, et al. Glucuronidation of statins in animals and humans: a novel mechanism of statin lactonization. Drug Metab Dispos. 2002;30(5):505–12.
Prueksaritanont T, Gorham LM, Ma B, Liu L, Yu X, Zhao JJ, et al. In vitro metabolism of simvastatin in humans [SBT]identification of metabolizing enzymes and effect of the drug on hepatic P450s. Drug Metab Dispos. 1997;25(10):1191–9.
Prueksaritanont T, Ma B, Yu N. The human hepatic metabolism of simvastatin hydroxy acid is mediated primarily by CYP3A, and not CYP2D6. Br J Clin Pharmacol. 2003;56(1):120–4.
Ramsey LB, Johnson SG, Caudle KE, Haidar CE, Voora D, Wilke RA, et al. The clinical pharmacogenetics implementation consortium guideline for SLCO1B1 and simvastatin-induced myopathy: 2014 update. Clin Pharmacol Ther. 2014. doi:10.1038/clpt.2014.125 [advance online publication].
Tsamandouras N, Dickinson G, Guo Y, Hall S, Rostami-Hodjegan A, Galetin A, et al. Identification of the effect of multiple polymorphisms on the pharmacokinetics of simvastatin and simvastatin acid using a population-modeling approach. Clin Pharmacol Ther. 2014;96(1):90–100.
Tsamandouras N, Rostami-Hodjegan A, Aarons L. Combining the “bottom-up” and “top-down” approaches in pharmacokinetic modelling: fitting PBPK models to observed clinical data. Br J Clin Pharmacol. 2013. doi:10.1111/bcp.12234.
Leil TA. A Bayesian perspective on estimation of variability and uncertainty in mechanism-based models. CPT: Pharmacosmet Syst Pharmacol. 2014;3:e121.
Gisleskog PO, Karlsson MO, Beal SL. Use of prior information to stabilize a population data analysis. J Pharmacokinet Pharmacodyn. 2002;29(5):473–505.
Langdon G, Gueorguieva I, Aarons L, Karlsson M. Linking preclinical and clinical whole-body physiologically based pharmacokinetic models with prior distributions in NONMEM. Eur J Clin Pharmacol. 2007;63(5):485–98.
Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28(5):481–504.
Bergstrand M, Karlsson M. Handling data below the limit of quantification in mixed effect models. AAPS J. 2009;11(2):371–80.
Boeckman AJ, Sheiner LB, Beal SL. NONMEM users guide - part VIII, help guide. Ellicott City: ICON Development Solutions; 2011.
Dokoumetzidis A, Aarons L. Analytical expressions for combining population pharmacokinetic parameters from different studies. J Biopharm Stat. 2008;18(4):662–76.
Pasanen MK, Neuvonen M, Neuvonen PJ, Niemi M. SLCO1B1 polymorphism markedly affects the pharmacokinetics of simvastatin acid. Pharmacogenet Genomics. 2006;16(12):873–9.
Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther. 2007;82(1):17–20.
Zhao P, Rowland M, Huang SM. Best practice in the use of physiologically based pharmacokinetic modeling and simulation to address clinical pharmacology regulatory questions. Clin Pharmacol Ther. 2012;92(1):17–20.
Agoram B. Evaluating systems pharmacology models is different from evaluating standard pharmacokinetic-pharmacodynamic models. CPT: Pharmacosmet Syst Pharmacol. 2014;3:e101.
Polli JW, Hussey E, Bush M, Generaux G, Smith G, Collins D, et al. Evaluation of drug interactions of GSK1292263 (a GPR119 agonist) with statins: from in vitro data to clinical study design. Xenobiotica. 2013;43(6):498–508.
Rowland Yeo K, Jamei M, Yang J, Tucker GT, Rostami-Hodjegan A. Physiologically based mechanistic modelling to predict complex drug-drug interactions involving simultaneous competitive and time-dependent enzyme inhibition by parent compound and its metabolite in both liver and gut—the effect of diltiazem on the time-course of exposure to triazolam. Eur J Pharm Sci. 2010;39(5):298–309.
Gertz M, Tsamandouras N, Sall C, Houston JB, Galetin A. Reduced physiologically-based pharmacokinetic model of repaglinide: Impact of OATP1B1 and CYP2C8 genotype and source of in vitro data on the prediction of drug-drug interaction risk. Pharm Res. 2014;31(9):2367-82.
Neuvonen PJ, Kantola T, Kivisto KT. Simvastatin but not pravastatin is very susceptible to interaction with the CYP3A4 inhibitor itraconazole. Clin Pharmacol Ther. 1998;63(3):332–41.
Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol. 2004;94(9):1140–6.
Son H, Lee D, Lim LA, Jang SB, Roh H, Park K. Development of a pharmacokinetic interaction model for co-administration of simvastatin and amlodipine. Drug Metab Pharmacokinet. 2014;29(2):120–8.
Satoh T, Taylor P, Bosron WF, Sanghani SP, Hosokawa M, Du BNL. Current progress on esterases: from molecular structure to function. Drug Metab Dispos. 2002;30(5):488–93.
Vree TB, Dammers E, Ulc I, Horkovics-Kovats S, Ryska M, Merkx I. Variable plasma/liver and tissue esterase hydrolysis of simvastatin in healthy volunteers after a single oral dose. Clin Drug Invest. 2001;21(9):643–52.
Tubic-Grozdanis M, Hilfinger J, Amidon G, Kim J, Kijek P, Staubach P, et al. Pharmacokinetics of the CYP 3A substrate simvastatin following administration of delayed versus immediate release oral dosage forms. Pharm Res. 2008;25(7):1591–600.
Gibiansky L, Gibiansky E, Bauer R. Comparison of Nonmem 7.2 estimation methods and parallel processing efficiency on a target-mediated drug disposition model. J Pharmacokinet Pharmacodyn. 2012;39(1):17–35.
Knauer MJ, Urquhart BL, Meyer zu Schwabedissen HE, Schwarz UI, Lemke CJ, Leake BF, et al. Human skeletal muscle drug transporters determine local exposure and toxicity of statins. Circ Res. 2010;106(2):297–306.
Kameyama Y, Yamashita K, Kobayashi K, Hosokawa M, Chiba K. Functional characterization of SLCO1B1 (OATP-c) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15 + C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet Genomics. 2005;15(7):513–22.
Tomita Y, Maeda K, Sugiyama Y. Ethnic variability in the plasma exposures of OATP1B1 substrates such as HMG-CoA reductase inhibitors: a kinetic consideration of its mechanism. Clin Pharmacol Ther. 2013;94(1):37–51.
Lippert J, Brosch M, von Kampen O, Meyer M, Siegmund HU, Schafmayer C, et al. A mechanistic, model-based approach to safety assessment in clinical development. CPT: Pharmacosmet Syst Pharmacol. 2012;1:e13.
Rose RH, Neuhoff S, Abduljalil K, Chetty M, Rostami-Hodjegan A, Jamei M. Application of a physiologically based pharmacokinetic model to predict OATP1B1-related variability in pharmacodynamics of rosuvastatin. CPT: Pharmacosmet Syst Pharmacol. 2014;3:e124.
Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, et al. SLCO1B1 variants and statin-induced myopathy-a genomewide study. N Engl J Med. 2008;359(8):789–99.
Watanabe T, Kusuhara H, Maeda K, Shitara Y, Sugiyama Y. Physiologically based pharmacokinetic modeling to predict transporter-mediated clearance and distribution of pravastatin in humans. J Pharmacol Exp Ther. 2009;328(2):652–62.
Chu X, Korzekwa K, Elsby R, Fenner K, Galetin A, Lai Y, et al. Intracellular drug concentrations and transporters: measurement, modeling, and implications for the liver. Clin Pharmacol Ther. 2013;94(1):126–41.
Fenneteau F, Poulin P, Nekka F. Physiologically based predictions of the impact of inhibition of intestinal and hepatic metabolism on human pharmacokinetics of CYP3A substrates. J Pharm Sci. 2010;99(1):486–514.
Lee AJ, Maddix DS. Rhabdomyolysis secondary to a drug interaction between simvastatin and clarithromycin. Ann Pharmacother. 2001;35(1):26–31.
Yeo KR, Yeo WW, Wallis EJ, Ramsay LE. Enhanced cholesterol reduction by simvastatin in diltiazem-treated patients. Br J Clin Pharmacol. 1999;48(4):610–5.
Gelman A, Bois F, Jiang J. Physiological pharmacokinetic analysis using population modeling and informative prior distributions. J Am Stat Assoc. 1996;91(436):1400–12.
Jonsson F, Jonsson EN, Bois FY, Marshall S. The application of a Bayesian approach to the analysis of a complex, mechanistically based model. J Biopharm Stat. 2007;17(1):65–92.
Krauss M, Burghaus R, Lippert J, Niemi M, Neuvonen P, Schuppert A, et al. Using Bayesian-PBPK modeling for assessment of inter-individual variability and subgroup stratification. In Silicon Pharmacol. 2013;1(1):6.
Wulkersdorfer B, Wanek T, Bauer M, Zeitlinger M, Muller M, Langer O. Using positron emission tomography to study transporter-mediated drug-drug interactions in tissues. Clin Pharmacol Ther. 2014;96(2):206–13.
ACKNOWLEDGMENTS AND DISCLOSURES
N.T. is the recipient of a PhD grant jointly awarded by the University of Manchester and Eli Lilly and Company. A.R-H. is an employee of the University of Manchester and parttime secondee to Simcyp Limited (a Certara Company). Simcyp’s research is funded by a consortium of pharma companies. The authors would like to acknowledge the fruitful comments and discussions made by Dr Michael Gertz, Roche and by the members of the Centre for Applied Pharmacokinetic Research at the University of Manchester. The authors would also like to thank Dr Joe Polli for the provision of individual SV and SVA data from Polli et al., 2013 [22].
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplement 1
(DOCX 714 kb)
Supplement 2
(DOCX 37 kb)
Supplement 3
(DOCX 145 kb)
Supplement 4
(DOCX 755 kb)
Supplement 5
(DOCX 27 kb)
Supplement 6
(DOCX 998 kb)
Supplement 7
(DOCX 2506 kb)
Supplement 8
(DOCX 31 kb)
Supplement 9
(DOCX 30 kb)
Rights and permissions
About this article
Cite this article
Tsamandouras, N., Dickinson, G., Guo, Y. et al. Development and Application of a Mechanistic Pharmacokinetic Model for Simvastatin and its Active Metabolite Simvastatin Acid Using an Integrated Population PBPK Approach. Pharm Res 32, 1864–1883 (2015). https://doi.org/10.1007/s11095-014-1581-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1581-2