Abstract
Avian coccidiosis is one of the most important diseases of poultry and it is responsible for a large number of all broiler mortalities worldwide. The control of this disease relies mainly on the use of anticoccidial drugs. However, herbal preparations could be an alternative for the treatment against coccidiosis in chickens. The direct effects of Moringa oleifera acetone extracts on broiler chickens naturally infected with mixed Eimeria species was investigated to determine the relative efficacy of the extracts against coccidiosis in birds. The investigations were carried out in seven groups (ten chickens per group). The birds were given various doses (1.0, 2.0, 3.0, 4.0 and 5.0 g/kg body weight) of acetone extract of leaves of M. toltrazuril® (positive control) and untreated (negative control). The extract was evaluated for anticoccidial activity by means of inhibition of oocyst output in faeces, faecal score, weight gain and mortality. Haematological indices were evaluated by standard methods. The group treated with 1.0 g/ kg body weight Moringa oleifera extract produced the least inhibitory effect on oocyst shed in the faeces (96.4 %), while the groups treated with 2.0 g/kg, 3.0 g/kg, 4.0 g/kg and 5.0 g/kg body weight of the extract produced 97.4, 98.7, 99.1 and 99.8 %, respectively. Body weight gains of infected chickens treated with the extract significantly improved (p < 0.05), and faecal scores were milder. Packed cell volume, haemoglobin concentration and red blood count of the treated birds were significantly (p < 0.05) higher than those of the infected untreated group. Moringa oleifera leaves could find application in the treatment of avian coccidiosis in veterinary practice.
Similar content being viewed by others
Introduction
Coccidiosis is ubiquitous where chickens are reared (traditional, industrial or organic/bio farms). It is recognised as the parasitic disease that has the greatest economic impact on poultry industries worldwide (Allen and Fetterer 2002) due to production losses and costs for treatment or prevention (Shirley et al. 2005). The global cost of the pathogen to the poultry industry is estimated to be over US$2.4 billion per annum (Shirley et al. 2005). These costs involve medication of the chickens, losses due to mortality, morbidity and poor growth of the chickens surviving the disease.
The intensive use of anticoccidial drugs has led to the development of resistance (Chapman 1997). Increasing development of drug-resistant coccidial species has stimulated searches for alternative control methods or new drugs. However, this has resulted in the increased cost of poultry products. Furthermore, drug or antibiotic residue in the poultry product is potentially offensive to the consumer. Therefore, the use of plant extracts as medicine may alleviate these problems, as they are not only natural products but also may comprise new therapeutic molecules to which resistance has not yet developed.
The use of herbal remedies in the management of coccidiosis is not a new concept. For example, halofuginone, a quinazolinone alkaloid derived from Dichroa febrifuga, has been used as a coccidiostat, and the original extract from D. febrifuga, known as febrifugine, possesses antimalarial and anticoccidial activity (Youn and Noh 2001). The investigation of herbal materials as anticoccidial remedies therefore holds promise as an alternative in the control of coccidiosis. In addition to possibly lowering the cost of food production in Nigeria, it could create a herbal remedy export market and thereby create more jobs in the country. The use of herbal extracts may also satisfy the increasing concerns of consumers, on condition that they prove to be both safe and effective. Marizvikuru et al. (2005) recorded that Aloe vera and Aloe spicata, Myrothamnus flabellifolia, Lannea stuhmannii, Ficus burkei (wild fig), Capsicum annum (Pepper), Parinari curatellifolia, Albizia gummisera and Albizia adianthifolia are common herbs used in Mushagashe, Zimbabwe to treat coccidiosis. Dietary Vernonia amygdalina and Azadirachta indica can inhibit invasion and/or replication of Eimeria in the gut tissues of chickens (Oyagbemi and Adejinmi 2012).
The Moringa tree grows mainly in semiarid tropical and subtropical areas. While it grows best in dry sandy soil, it tolerates poor soil, including coastal areas. It is a fast-growing, drought-resistant tree that is native to the southern foothills of the Himalayas and possibly Africa and the Middle East. Today it is widely cultivated in Africa, Central and South America, Sri Lanka, India, Mexico, Malaysia and the Philippines. Considered one of the world’s most useful trees, as almost every part of the Moringa tree can be used for food, or has some other beneficial property. In the tropics it is used as foliage for livestock.
Farooq et al. (2007) documented M. oleifera Lam (Moringaceae) as a highly valued plant, distributed in many countries of the tropics and subtropics. It has an impressive range of medicinal uses with high nutritional value. Different parts of this plant contain a profile of important minerals and are a good source of protein, vitamins, β carotene, amino acids and various phenolics. The Moringa plant provides a rich and rare combination of zeatin, quercetin, β - sitosterol, caffeoylquinic acid and kaempferol. In addition to its compelling water purifying powers and high nutritional value, M. oleifera is very important for its medicinal value. Various parts of this plant such as the leaves, roots, seed, bark, fruit, flowers and immature pods act as cardiac and circulatory stimulants, antioxidant, antibacterial, antimalarial and antifungal activities, and are being employed for the treatment of different ailments in the indigenous system of medicine, particularly in South Asia (Farooq et al. 2007). The acute oral toxicity study in male Wistar albino mice estimated the LD50 of M. oleifera leaves to be 1,585 mg/kg (Awodele et al. 2012). No studies have examined the anticoccidial efficacy of the plant in livestock; thus, this study has provided evidence for the efficacy of M. oleifera and its positive effect on the haematological parameters in broiler chickens naturally infected with field strains of Eimeria species.
Materials and methods
Parasites
Birds were naturally infected with field strains of Eimeria species. The field isolates contained the following species (differentiated on the basis of oocyst morphology, site of colonisation, pathology and clinical signs): E. tenella, Eimeria acervulina, Eimeria necatrix, Eimeria maxima and Eimeria brunetti.
Collection of plant and extraction
M. oleifera leaves were obtained from Ibadan, southwestern Nigeria, in the months of May and June 2012 and identified and authenticated in the herbarium of the Department of Botany, University of Ibadan, Nigeria, where a voucher specimen was deposited with voucher number UIH22346. The leaves were air-dried. The dried leaves were reduced to a fine powder by grinding with a milling machine. Dried and grinded M. oleifera leaves were extracted. Extracts were prepared by maceration with intermittent shaking for 72-h in 70 % acetone with a 10:1 solvent to dry weight ratio (Eloff 1998). The extracts were filtered using Whatman no 1 filter paper using a funnel, and the acetone removed under stream of air and stored in dark container at 4 °C until use.
Birds and experimental design
A total of 70-day old Anak broiler chickens (Zartech®) of both sexes were reared as a single group for 2 weeks. On the onset of clinical signs typical of coccidiosis (McDougald and Reid 1997), following natural infection, thebirds were randomly assigned to seven treatments groups (A-G) according to the oocyst level in their faeces. Group A was the untreated group (negative control), and group B (positive control) was treated with toltrazuril (Vazuril®, Jordan) orally as prescribed by the manufacturer (7 mg/kg) for 2 days. Groups C G were individually drenched with 1.0 to 5.0 g/kg body weight of acetone leaf extract of M. oleifera respectively once a day for 5 days. A treatment period of 5 days was selected as this was the estimated period of oxidant insult induced by the coccidian parasite (Koinarski et al. 2005). The preliminary acute toxicity study was conducted by using 25, day-old broiler chickens which were divided into five groups of five chickens each. Each bird in groups A-E was individually drenched with the graded doses of the acetone extract of M. oleifera to be tested. The chickens were observed for 24 h for any signs of toxicity including change in behaviour or death.
Diets and management
The birds were fed ad libitum on a proprietary broiler ration (Top feeds®, Broiler rations) without coccidiostat additives and also given access to water ad libitum. They were routinely vaccinated against Newcastle disease and infectious bursa disease. The birds were kept in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals.
Data collection
Oocyst output in the faeces: To measure output the of oocysts in the faeces, pooled daily oocyts counts per group of birds, were undertaken as previously described by Holdsworth et al. (2004). Individual oocyst counts were not feasible because the birds were housed in groups. A total of 10 g of faeces was collected daily beginning from a day before treatment until the end of the treatment period and was processed. The modified McMaster technique as described by Vassilev (2002) was used to estimate the oocysts per gram (OPG). The changes in OPG over time were evaluated using the paired sample t test.
Growth performance (weight gain): To measure growth performance as gain of weight, birds from each treatment group were weighed individually on a daily basis throughout the treatment period. Individual and mean body weight gains were calculated. Differences in the average weights per group for each time point were compared using paired sample t-test to determine if a significant difference was present between the treatment groups.
The mortality was determined using the following formula: percent mortality = (total number of dead chicks in the group/initial number of birds in group) × 100 and evaluated for death rate and hazard analysis after being exposed to the infectious agent in the absence and presence of the herbal extract.
Following the onset of clinical signs, the extent of bloody/tan diarrhoeal score was assigned one of the four degrees, from 0(−) to 3(+++). Zero was the normal status, whereas 1, 2, and 3 corresponded to 33, 33–66, and 66–99 % blood/tan colour in total faeces, respectively. This was done according to the method described by Youn et al. (1993) and Rao Zahid et al., (2010).
Blood samples (2 ml) were collected from the wing venipuncture from three chickens in each group at the onset of the study, day 3 of the study and after the study. The packed cell volume (PCV) was determined by microhaematocrit method (Goldenfarb et al. 1971). The values found were expressed as a percentage of the total blood volume. Red blood cell (RBC) and white blood cells (WBC) were counted using haemocytometer (Jain 1986). Haemoglobin (Hb) concentration was determined by standard cyanometahemoglobin method as described by Jain (1986) and its values were expressed in gram per millilitre of blood.
Statistics
The data obtained from the study were summarised as means ± standard deviation of means. All statistical analysis was undertaken using the programme SPSS 13 (SPSS Inc.). Differences in the average weights per group for each time point were compared using ANOVA to determine if a significant difference was present between the treatment groups. Weight gains for each group for the different time points were compared using a paired sample t-test to ascertain if weights differed significantly over the growth period.
Results
The acetone leaf extract of M. oleifera gave a yield of 184.43 g (17.37 % w/w). The preliminary acute toxicity test conducted revealed that there was no sign of toxicity (eye blinking, panting, lethargy, salivation and incoordination) or mortality in any of the treated groups of broiler chicken including those drenched with the highest dose (5.0 g/ kg body weight) of M. oleifera acetone leaf extract.
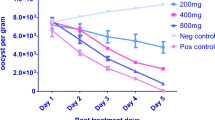
The acetone extract of M. oleifera demonstrated an inhibitory effect on the shedding of oocyst in faeces in boiler chickens in a concentration dependent manner (Fig. 1). The group treated with toltrazuril gave the highest oocyst inhibitory effect on oocyst shed in faeces (100 %), although not to a significant level (p > 0. 05) compared with the groups treated with M. oleifera. In the untreated group (control), there was no inhibitory effect on oocyst shed in faeces instead an increase in its shedding. There was a significant difference (P < 0.05) in oocyst inhibitory effect between the untreated group and the treated groups. The group treated with 1.0 g/ kg body weight of acetone extract of Moringa oleifera produced the least inhibitory effect on oocyst shed in faeces (96.4 %), while the groups treated with 2.0, 3.0, 4.0 and 5.0 g/kg body weight of the extract produced 97.4, 98.7, 99.1 and 99.8 % inhibition of oocyst shed in the faeces respectively.
Changes in body weight of broiler chickens before, during and after the treatment are shown in Fig. 2. The untreated group had 14.0 % reduction in body weight, the group treated with toltrazuril had 27.0 % increase in body weight, while the group treated with 1.0 g/kg, 2.0, 3.0, 4.0 and 5.0 g/kg body weight of acetone extract of leaves of Moringa oleifera gave 20.0, 22.0, 26.0, 29.0 % and 34.0 % body weight gain respectively. There was a significant difference (P < 0.05) in weight gain between the untreated group and the treated groups. The result showed that the group treated with 5.0 g/kg body weight of the extract produced the highest weight gain. The group treated with 5.0 g/kg body weight of the extract shows a significant weight difference (p < 0.05) compared with the untreated group and the groups treated with 1.0, 2.0 and 3.0 g/kg body weight of M. acetone extract, however there was no significant difference (p > 0.05) between the group treated with 5.0 g/kg body weight and the groups treated with toltrazuril and 4.0 g/kg body weight of the extract.
One bird died due to coccidial infection on days 6 and 7 in the untreated group and on day 5 in the group treated with 1.0 g of M. oleifera acetone extract.
Bloody/tan coloured diarrhoeal were observed in all the treatment groups at the onset of treatment as shown in Table 1. The degree of bloody / tan colour diarrhea in broiler chickens treated with toltrazuril was milder compared with groups treated with leaves of M. oleifera extract; however, the mildness observed in the groups treated with the extract of M. oleifera was in a concentration dependent manner.
The mean packed cell volume, haemoglobin concentration and red blood cell count after treatment of the broiler chickens with acetone leaf extract of M. oleifera was significantly higher (p < 0.05) than the untreated groups, while the white blood cell count was significantly higher in the untreated broiler chickens than the treated groups.
Discussion
The preliminary acute toxicity carried out in our study using M. oleifera, showed no sign of acute toxicity. The use of M. oleifera is justified on the basis that it possesses anti-malaria properties (Farooq et al.,2007), and Eimeria belongs to the phylum Apicomplexa as does the Plasmodium parasite. The result of the oocyst inhibition in the faeces shows that the group treated with 5.0 g/kg body weight of leaves of M. oleifera extract was most promising with 99.8 % inhibition of Eimeria oocyst shed in the faeces on the seven day of the study. However statistically, paired sample t-test suggested the absence of significant difference with the other treated groups. The inhibition of oocyst shed in faeces could be attributed to the antioxidant properties possessed by M. oleifera. According to Allen et al. (1998) antioxidant compounds are known to reduce the severity of E. tenella infections by ameliorating the degree of intestinal lipid peroxidation. The antioxidant properties of M. oleifera have earlier been reported by Siddhuraju and Becker (2003) and Dillard and German 2000. The antioxidant constituents of M. oleifera are ascorbic acid, flavonoids, phenolics and carotenoid (Anwar et al. 2005; Siddhuraju and Becker 2003; Dillard and German 2000). However, the mechanism of the anticoccidial actions still needs to be explained. The synthetic anticoccidial toltrazuril indicated a much higher oocyst inhibition, although not significantly different with other treatment groups. The better anticoccidial effect of toltrazuril could be attributed to the fact that toltrazuril is a pure active substance, while the extracts contain several chemical compounds. Naidoo et al. (2008) used Artemisia afra leaves at 150 mg/kg body weight and obtained 66.7 % oocyst reduction on day 7 of their study while Oyagbemi and Adejinmi (2012) used 10 % of V. amygdalina leaves as supplement in broiler feed and observed 99.5 % oocyst reduction at day 7 of their study. Abbas et al. (2010) used 3 % of Curcuma longa leaves as supplement in broiler feed and got 94.7 % oocyst inhibition rate at day 7 of their study.
The acetone extract of M. oleifera leaves at different concentration had a good dose related response to the improvement of body weight gain in broiler chickens infected with mixed species of Eimeria. On the seven day of the study, the group treated with 5.0 g/kg body weight of the extract demonstrated 33.6 % body weight gain while the groups treated with 4.0, 3.0, 2.0 and 1.0 g/kg body weight had 29.5, 26.1, 22.2, and 19.5 %, body weight gain respectively. The group treated with toltrazuril had 27.2 % body weight gain. There was no significant difference in the body weight gain between the group treated with 5.0 g/kg body weight and the group treated with 4.0 g/kg body weight, however, there was a significant difference in body weight gain between the group treated with 5.0 g/kg body weight and the other treatment groups. Our result shows that the untreated group had 14.0 % weight loss; this could be as a result of the damaging effect of the Eimeria species on the epithelial lining of the intestines which in turn impairs the absorption of nutrient and water from the intestine. The weight gain observed in the groups treated with M. oleifera leaves could also be due to its nutritional value, having crude protein of 17.01 %, carbohydrate (63.11 %), crude fat (2.11 %) and energy (1440.11 kcal/100 g) according to Ogbe and John (2012). However, Booth and Wickens (1988) and Oduro et al. (2008) reported that M. oleifera leaf meal has 27.1 and 27.51 % crude protein respectively. The improvement of the body weight could as well be attributed to the high levels of vitamins and amino acids in Moringa oleifera leaves which improve recovery from the disease condition and increase feed intake and feed conversion rate.
Bloody/tan coloured diarrhoeal faeces were seen in all the groups following the onset of clinical signs in infected broiler chickens. The bloody/tan coloured diarrhoea was milder in all the treatment (toltrazuril and acetone extract of M. oleifera) groups as the days progressed. This can be attributed to the reduction of oocyst shed in the faeces of the broiler chickens as the day progressed. The reduction of oocyst shed in the faeces will result in reduced damage caused on the intestinal lining and as a result the reduction in the level of haemorrhage. Rao Zahid et al. (2010) also observed a reduction in the level of bloody diarrhea in broiler chickens infected with E. tenella after treatment with C. longa leaves. On the four day of our study, the faeces of the birds treated with 5.0 g/kg body weight of M. oleifera were devoid of diarrhoeal blood/ tan colour. Our findings showed that the haematological parameters (PCV, Hb and RBC) of the infected broiler chickens was lower than the normal values prior to treatment, while an increase in WBC count was observed. This is due to the lost of blood in the intestine of the birds caused by the coccidial parasite. Ellakany et al. (2011) also observed a significant decrease in packed cell volume, haemoglobin concentration and lymphocyte percentage of E. tenella infected broilers. However the broiler chickens treated with acetone extract of M. oliefera had an increase in the values of their haematological parameters post treatment. The improvements of haematological parameter could be attributed to the daily reduction of oocyst as well as the haemopoietic effect of M. oleifera reported by Estrella et al. (2000) and Siddhuraju and Becker (2003).
With Moringa oleifera extracts promoting a similar OPG reduction and weight gain to toltrazuril, the use of the plant may be of benefit in the management of coccidiosis. This provides a strong rationale for further evaluation of the anticoccidial efficacy of M. oleifera plant extracts in a larger study. This plant may also have merit as feed additive in the prophylactic management of coccidiosis.
Conclusion
The present study confirms that M. oleifera possesses anticoccidial property. Since M. oleifera is reported to be safe and widely available and could be easily cultivated, it could, therefore, serve as a useful alternative product for the control of avian coccidiosis in poultry production. Further in vitro and in vivo investigations into the isolated fractions should be conducted to investigate the potential therapeutic use of this plant. There could be more than one active principle that induced the observed anticoccidial activity in the plant.
References
Abbas, R.Z., Iqbal, Z., Khan, M.N., Zafar, M.A., and Zia, M.A. 2010. Anticoccidial Activity of Curcuma longa L. in Broilers. Brazil Archival Biology and Technology 53(1), 63–67.
Allen, P.C. and Fetter R.H., 2002. “Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry.” Clinical Microbiology Review 15, 58–65
Allen, P.C., Danforth, H.D. and Augustine, P.C. 1998. Dietary modulation of avian coccidiosis. Interenational Journal of Parasitology 28, 1131–1140.
Anwar, F., Ashraf, M. and Bhanger M.I. 2005. Interprovenance variation in the composition of Moringa oleifera oilseeds from Pakistan. Journal American Oil Chemical Society 82, 45–51.
Awodele, O., Oreagba, I.A., Odoma S., da Silva, J.A. and Osunkalu, V.O. 2012. Toxicological evaluation of the aqueous leaf extract of Moringa oleifera Lam. (Moringaceae). Journal of Ethnopharmacology 139(2), 330–336
Booth, F.E.M. and Wickens, G.E., 1988. Non-timber uses of selected arid zone trees and shrubs in Africa. FAO conservation guide, Rome, pp 92–101.
Chapman, H.D. 1997. “Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl,” Avian Pathology 26, 221–244.
Dillard, C.J. and German, J.B. 2000. Phytochemicals: nutraceuticals and human health: A review. Journal of Science, Food and Agriculture 80, 1744–1756.
Ellakany, H.F., Abuakkada, S.S., Oda, S.S. and El-Sayed Y.S. 2011. Influence of low levels of dietary aflatoxins on Eimeria tenella infections in broilers. Tropical Animal Health and Production 43, 249–257.
Eloff, J.N. 1998. Which extract should be used for the screening and isolation of antimicrobial components from plants. Journal of Ethnopharmacol., 60, 1–8.
Estrella, M.C.P., Mantaring, J.B.V. and David, G.Z. 2000. A double blind, randomised controlled trial on the use of malunggay (Moringa oleifera) for augmentation of the volume of breast milk among non-nursing mothers of preterm infants. Philippine Journal of Pediatrician 49, 3–6.
Farooq, A., Sajid, L., Muhammad, A. and Anwarul, H. G. 2007. Moringa oleifera: A Food Plant with Multiple Medicinal Uses. Phytotherapy Research 21, 17–25.
Goldenfarb, P.B., Bowyer, F.P., Hall, T. and Brosious, E. 1971. Reproducibility in the hematology laboratory: the microhematocrit determination, American Journal Clinical Pathology 52: 35–36.
Holdsworth, P.A., Conway, D.P., McKenzie, M.E., Dayton, A.D., Chapman, H.D., Mathis, G.F., Skinner, J.T., Mundt, H.C. and Williams, R.B. 2004. World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys, Veterinary Parasitology 121, 189–212.
Jain, N.C. 1986. Schalm's veterinary haematology. Lea and Febiger, Schalm's Veterinary Haematology, 4th ed. Philadelphia: Lea. Febiger, pp. 71–74.
Koinarski, V., Georgieva, N., Gadjeva, V. and Petkov, P. 2005. Antioxidant status of broiler chickens infected with Eimeria acervulina. Review Médical Véterinari 156 (10), 498–502.
Marizvikuru, M., Evison, B., Michael, C. and Tinyiko, E. H. 2005. Use of Herbal Plants in Poultry Health Management in the Mushagashe Small-Scale Commercial Farming Area in Zimbabwe. International Journal of Applied Research in Veterinary Medicine 3, (2), 163–170
McDougald, L.R. and Reid, W.M. 1997. Coccidiosis. In: B.W. Calnek(Eds). Diseases of Poultry. 10th Ed. (Iowa State University Press, Ames), pp 865–883.
Naidoo, V., McGaw, L.J., Bisschop, S.P.R., Duncan, N. and Eloff, J.N. 2008. The value of plant extracts with antioxidant activity in attenuating coccidiosis in broiler chickens. Veterinary Parasitology 153, 214–219.
Oduro, I., Ellis, W.O. and Owusu, D. 2008. Nutritional potential of two leafy vegetables: Moringa Oleifera and Impomea batatas leaves. Science Research Essay 3(2), 57–60.
Ogbe A.O. and John P. A. 2012. Effect of Polyherbal Aqueous Extracts (Moringa oleifera, Gum arabic and wild Ganoderma lucidum) in Comparison with Antibiotic on Growth Performance and Haematological Parameters of Broiler Chickens. Research Journal of Recent Science 1(7), 10–18.
Oyagbemi, T.O. and Adejinmi, J.O. 2012. Supplementation of broiler feed with leaves of Vernonia amygdalina and Azadirachta indica protected birds naturally infected with Eimeria sp. African Journal of Biotechnology 11(33), pp. 8407–8413.
Rao Zahid, A., Zafar, I., Muhammad, N. K., Muhammad, A. Z. and Muhammad, A. Z. 2010. Anticoccidial Activity of Curcuma longa L. in Broilers. Brazilian Archives of Biology and Technology 53, 63–67,
Shirley, M.W., Smith, A.L., and Tomley, F.M. 2005. The biology of avian Eimeria with an emphasis on their control by vaccination. Advance Parasitology 60, 285–330.
Siddhuraju, P. and Becker, K. 2003. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agro-climatic origins of drumstick tree (Moringa oleifera Lam.). Journal of Agriculture and Food Chemistry 15, 2144–2155.
Vassilev, D.G. 2002. Special modification of Mc Master, In: B. Cumming (ed), Manual of veterinary parasitological techniques. (Central Veterinary Laboratory, London).
Youn, H.J. and Noh, J.W. 2001. Screening of the anticoccidial effects of herb extracts against Eimeria tenella. Veterinary Parasitology 96, 257–263.
Youn, H.J., Kang, Y.B. and Jang, D.H. 1993. Effects of γ-irradiation from cobalt-60 on pathogenicity of Eimeria tenella. Korean Journal of Veterinary Research, 33, 649–655.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ola-Fadunsin, S.D., Ademola, I.O. Direct effects of Moringa oleifera Lam (Moringaceae) acetone leaf extract on broiler chickens naturally infected with Eimeria species . Trop Anim Health Prod 45, 1423–1428 (2013). https://doi.org/10.1007/s11250-013-0380-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-013-0380-9