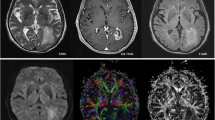
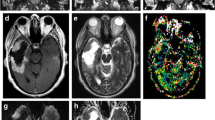
Abstract
As the research on cellular changes has shed invaluable light on the pathophysiology and biochemistry of brain tumors, clinical and experimental use of molecular imaging methods is expanding and allows quantitative assessment. The term molecular imaging is defined as the in vivo characterization and measurement of biologic processes at the cellular and molecular level. Molecular imaging sets forth to probe the molecular abnormalities that are the basis of disease rather than to visualize the end effects of these molecular alterations and, therefore, provides different additional biochemical or molecular information about primary brain tumors compared to histological methods “classical” neuroradiological diagnostic studies. Common clinical indications for molecular imaging contain primary brain tumor diagnosis and identification of the metabolically most active brain tumor reactions (differentiation of viable tumor tissue from necrosis), prediction of treatment response by measurement of tumor perfusion, or ischemia. The interesting key question remains not only whether the magnitude of biochemical alterations demonstrated by molecular imaging reveals prognostic value with respect to survival, but also whether it identifies early disease and differentiates benign from malignant lesions. Moreover, an early identification of treatment success or failure by molecular imaging could significantly influence patient management by providing more objective decision criteria for evaluation of specific therapeutic strategies. Specially, as molecular imaging represents a novel technology for visualizing metabolism and signal transduction to gene expression, reporter gene assays are used to trace the location and temporal level of expression of therapeutic and endogenous genes. Molecular imaging probes and drugs are being developed to image the function of targets without disturbing them and in mass amounts to modify the target’s function as a drug. Molecular imaging helps to close the gap between in vitro and in vivo integrative biology of disease.
Similar content being viewed by others
References
Beaumont A, Whittle IR (2000) The pathogenesis of tumor associated epilepsy. Acta Neurochir 142:1–15
Schaller B (2003) Neuroprotection in brain tumors—pathophysiological sense or nonsense? Nervenarzt 74:1134–1136
Schaller B (2005) Influences of brain tumor-associated pH changes and hypoxia on epileptogenesis. Acta Neurol Scand 111:75–83
Schaller BJ, Buchfelder M (2006) Neuroprotection in primary brain tumors: Sense or nonsense? Expert Rev Neurother 6:723–730
Weissleder R, Mahmood U (2001) Molecular imaging. Radiology 219:316–333
Barker FG, Israel MA (1999) Molecular genetics. In: Berger MS, Wilson CB (eds) The gliomas. Philadelphia: W.B. Saunders Co, pp 39–51
Ichimura K, Bolin MB, Goike HM, et al. (2000) Deregulation of the p14ARF/MDM2/p53 pathway is a prerequisite for human astrocytic gliomas with G1-S transition control gene abnormalities. Cancer Res 60:417–424
Jacobs AH, Kracht LW, Gossmann, et al. (2005) Imaging in neurooncology. NeuroRx 2:333–347
Morrison RS (1999) Growth factor mediated signaling pathways. In: Berger MS, Wilson CB (eds) The gliomas. Philadelphia: W.B. Saunders, pp 52–64
Kleihues P, Burger PC, Collins VP, et al. (2000) Glioblastoma. In: Kleihues P, Cavenee WK (eds) Pathology and genetics of tumours of the nervous system. World Health Organization Classification of Tumours. Lyon: IARC Press, pp 29–39
Lang FF, Miller DC, Koslow M, et al. (1994) Pathways leading to glioblastoma multiforme: A molecular analysis of genetic alterations in 65 astrocytic tumors. J Neurosurg 81:427–436
Cairncross JG, Ueki K, Zlatescu MC, et al. (1998) Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 90:1473–1479
DeAngelis LM, Burger PC, Green SB, et al. (1998) Malignant glioma: Who benefits from adjuvant chemotherapy? Ann Neurol 44:691–695
Reifenberger G, Louis DN (2003) Oligodendroglioma: Toward molecular definitions in diagnostic neuro-oncology. J Neuropathol Exp Neurol 62:111–126
Sasaki H, Zlatescu MC, Betensky RA, et al. (2002) Histopathological-molecular genetic correlations in referral pathologist-diagnosed low-grade “oligodendroglioma.” J Neuropathol Exp Neurol 61:58–63
Buonocore E (1992) Comparison of PET with conventional imaging techniques, in clinical positron emission tomography. St. Louis, MO: Mosby-Year Book, pp 17–2
Del Sole A, Falini A, Ravasi L, et al. (2001) Anatomical and biochemical investigation of primary brain tumours. Eur J Nucl Med 28:1851–1872
Jasanoff A (2005) Functional MRI using molecular imaging agents. Trends Neurosci 28:120–126
Schaller B (2004) Usefulness of positron emission tomography in diagnosis and treatment follow-up of brain tumors. Neurobiol Dis 15:437–448
Blasberg RG, Tjuvajev JG (2003) Molecular-genetic imaging: Current and future perspectives. J Clin Invest 111:1620–1629
Heiss WD, Pawlik G, Herholz K, et al. (1984) Regional kinetic constants and cerebral metabolic rate for glucose in normal human volunteers determined by dynamic positron emission tomography of [18F]-2-fluoro-2-deoxy-d-glucose. J Cereb Blood Flow Metab 4:212–223
Phelps ME (2000) PET: The merging of biology and imaging into molecular imaging. J Nucl Med 41:661–681
Sokoloff L, Reivich M, Kennedy C, et al. (1977) The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28:897–916
Derlon JM, Borudet C, Bustany P, et al. (1989) (11C)L-methionine uptake in gliomas. Neurosurgery 25:720–728
Patronas NJ, DiChiro G, Kuftas C, et al. (1985) Prediction of survival in glioma patients by means of positron emission tomography. J Neurosurg 62:816–822
Ogawa T, Shishido F, Kanno I, et al. (1993) Cerebral glioma: Evaluation with methionine PET. Radiology 186:45–53
Mosskin M, Bergstrom M, Collins VP, et al. (1986) Positron emission tomography with 11C-methionione of intracranial tumors compared with histology of multiple biopsies. Acta Radiol Suppl 369:157–160
Kaschten B, Stevenaert A, Sadzot B, et al. (1998) Preoperative evaluation of 54 gliomas by PET with fluorine-18-fluorodeoxyglucose and/or carbon-11-methionine. J Nucl Med 39:778–785
Roelcke U, Leenders KL (1999) Positron emission tomography in patients with primary CNS lymphomas. J Neuro-Oncol 43:231–236
Pruim J, Wilemsen AT, Molenaar WM, et al. (1995) Brain tumors: L-(11C)tyrosine PET for visualization and quantification of protein synthesis rate. Radiology 197:221–226
Wienhard K, Herholz K, Voges J, et al. (1991) Increased amino acid transport into brain tumors measured by PET of L-(218F)fluorotyrosine. J Nucl Med 32:1338–1346
DeWolde H, Pruim J, Mastik MF, et al. (1997) Proliferative activity in human brain tumors: Comparison of histopathology and L-(1-11C)tyrosine PET. J Nucl Med 38:1369–1374
Kracht LW, Friese M, Herholz K, et al. (2003) Methyl-[11C]-l-methionine uptake as measured by positron emission tomography correlates to microvessel density in patients with glioma. Eur J Nucl Med Mol Imaging 30:868–873
Kole AC, Plaat BE, Hockstra HJ, et al. (1999) FDG and L-(1-11C)-tyrosine imaging of soft-tissue tumors before and after therapy. J Nucl Med 40:381–386
Wienhard K, Pawlik G, Nebeling B, et al. (1991) Estimation of local cerebral glucose utilization by positron emission tomography: Comparison of [18F]2-fluoro-2-deoxy-d-glucose and [18F]2-fluoro-2-deoxy-d-mannose in patients with focal brain lesions. J Cereb Blood Flow Metab 11:485–491
Sato N, Suzuki M, Kuwata N, et al. (1999) Evaluation of the malignancy of glioma using 11C-methione positron emission tomography and proliferating cell nuclear antigen staining. Neurosurg Rev 22:210–214
Nyberg G, Bergstrom M, Enblad P, et al. (1997) PET methionine of skull base neuromas and meningiomas. Acta Oto-laryngol 117:482–489
Herholz K, Rudolf J, Heiss WD (1992) FDG transport and phosphorylation in human gliomas measured with dynamic PET. J Neuro-Oncol 12:159–165
Bauer A, Langen KJ, Bidmer H, et al. (2005) 18F-CPFPX PET identifies changes in cerebral A1 adenosine receptor density caused by glioma invasion. J Nucl Med 46:450–454
Bergstrom M, Collins VP, Ehrin E, et al. (1983) Discrepancies in brain tumor extend as shown by computed tomography and positron emission tomography using (68Ga)EDTA (11C)glucose, and (11C)methionine. J Comput Assist Tomogr 7:1062–1066
Grosu AL, Weber WA, Riedel E, et al. (2005) L-(methyl-11C) methionine positrone emission tomogrpahy for target delineation in resected high-grade gliomas bifore radiotherapy. Int J Radiat Oncol Biol Phys 63:64–74
Mineura K, Sasajima T, Kowada M, et al. (1991) Innovative approach in the diagnosis of gliomatosis cerebri using carbon-11 L-methionine positron emission tomography. J Nucl Med 32:726–728
Duncan JD, Moss SD, Bandy DJ, et al. (1997) Use of positron emission tomography for presurgical localization of eloquent brain areas in children with seizures. Pediatr Neurosurg 26:144–156
Choi SJ, Kim JS, Kim JH, et al. (2006) 18F-3-deoxy-3-fluorothymidine PET for the diagnosis and grading for tumors. Eur J Nucl Med Mol Imaging (in press)
Goldman S, Levivier M, Piorotte B, et al. (1997) Regional methionine and glucose uptake in high-grade gliomas: A comparative study on PET-guided stereotactic biopsy. J Nucl Med 38:1459–1462
Go KG, Keuter EJ, Kamman RL, et al. (1994) Contribution of magnetic resonance spectroscopic imaging and L-(1-11C)tyrosine positron emission tomography to localization of cerebral gliomas for biopsy. Neurosurgery 34:994–1102
Costa DC, Gacinovic S, Miller RF (1995) Radionuclide brain imaging in acquired immunodeficiency syndrome (AIDS). Q J Nucl Med 36:243–249
Wurker M, Herholz K, Voges J, et al. (1996) Glucose consumption and methionine uptake in low-grade gliomas after iodone-125 brachytherapy. Eur J Nucl Med 23:583–586
Hoffman JM, Hanson MW, Friedman HS, et al. (1992) FDG-PET in pediatric posterior fossa brain tumors. J Comput Assist Tomogr 16:62–68
Kaplan AM, Bandy DJ, Manwaring KH, et al. (1999) Functional brain mapping using positron emission tomography scanning in preoperative neurosurgical planning for pediatric brain tumors. J Neurosurg 91:797–803
Utriainen M, Metsahonkala L, Salmi TT, et al. (2002) Metabolic characterization of childhood brain tumors: Comparison of 18F-flurodeoxyglucose and 11C-methionine positron emission tomography. Cancer 95:1376–1388
Jacobs A, Tjuvajev JG, Dubrovin M, et al. (2001) Positron emission tomography-based imaging of transgene expression mediated by replication—conditional, oncolytic herpes simplex virus type 1 mutant vectors in vivo. Cancer Res 61:2983–2995
Luciganini G, Losa M, Moresco RM, et al. (1997) Differentiation of clinically non-functioning pituitary adenomas from meningiomas and craniopharyngiomas by positron emission tomography wit (18F)fluoro-ethyl-spiperone. Eur J Nucl Med 24:1149–1155
Jacobs AH, Dittmar C, Winkeler A, et al. (2002) Molecular imaging of gliomas. Mol Imaging 1:309–335
Su H, Forbes A, Gambhir SS, et al. (2004) Quantization of cell number by a positron emission tomography reporter gene strategy. Mol Imaging Biol 6:139–148
Doubrovin M, Ponomarev V, Beresten T, et al. (2001) Imaging transcriptional regulation of p53-dependent genes with positron emission tomography in vivo. Proc Natl Acad Sci U S A 98:9300–9305
Serganova I, Doubrovin M, Vider J, et al. (2004) Molecular imaging of temporal dynamics and spatial heterogeneity of hypoxia-inducible factor-1 signal transduction activity in tumors in living mice. Cancer Res 64:6101–6108
Uhrbom L, Nerio E, Holland EC (2004) Dissecting tumor maintenance requirements using bioluminescence imaging of cell proliferation in a mouse glioma model. Nat Med 10:1257–1260
Wen B, Burgman P, Zanzonico P, et al. (2004) A preclinical model for noninvasive imaging of hypoxia-induced gene expression; comparison with an exogenous marker of tumor hypoxia. Eur J Nucl Med Mol Imaging 31:1530–1538
Anderson SA, Glod J, Arbab AS, et al. (2004) Non-invasive MR imaging of magnetically labeled stem cells to directly identify neovasculature in a glioma model. Blood 105:420–425
Chen X, Park R, Shahinian AH, et al. (2004) 18F-labeled RGD peptide: Initial evaluation for imaging brain tumor angiogenesis. Nucl Med Biol 31:179–189
Haubner R, Wester HJ, Weber WA, et al. (2001) Noninvasive imaging of α(v)ß3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res 61:1781–1785
Sundaresan G, Yazaki PJ, Shively JE, et al. (2003) 124I-labeled engineered anti-CEA minibodies and diabodies allow high-contrast, antigen-specific small-animal PET imaging of xenografts in athymic mice. J Nucl Med 44:1962–1969
van Waarde A, Buursma AR, Hospers GA, et al. (2004) Tumor imaging with 2 δ-receptor ligands, 18F-FE-SA5845 and 11C-SA4503: A feasibility study. J Nucl Med 45:1939–1945
Grohn OH, Valonen PK, Lehtimaki KK, et al. (2003) Novel magnetic resonance imaging contrasts for monitoring response to gene therapy in rat glioma. Cancer Res 63:7571–7574
Jacobs AH, Winkeler A, Hartung M, et al. (2003) Improved HSV-1 amplicon vectors for proportional coexpression of PET marker and therapeutic genes. Hum Gene Ther 14:277–297
Ponomarev V, Doubrovin M, Serganova I, et al. (2004) A novel triple-modality reporter gene for whole-body fluorescent, bioluminescent, and nuclear noninvasive imaging. Eur J Nucl Med Mol Imaging 31:740–751
Hamstra DA, Lee KC, Tychewicz JM, et al. (2004) The use of 19F spectroscopy and diffusion-weighted MRI to evaluate differences in gene-dependent enzyme prodrug therapies. Molec Ther 10:916–928
Mamot C, Nguyen JB, Pourdehnad M, et al. (2004) Extensive distribution of liposomes in rodent brains and brain tumors following convection-enhanced delivery. J. Neuro-oncol 68:1–9
Saito R, Bringas JR, McKnight TR, et al. (2004) Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res 64:2572–2579
Rehemtulla A, Stegman LD, Cardozo SJ, et al. (2000) Rapid and quantitative assessment of cancer treatment response using in vivo bioluminescence imaging. Neoplasia 2:491–495
Ross BD, Chenevert TL, Garwood M, et al. (2003) Evaluation of (E)-2′-deoxy-2′-(fluoromethylene)cytidine on the 9L rat brain tumor model using MRI. NMR Biomed 16:67–76
Rubin JB, Kung AL, Klein RS, et al. (2003) A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A 100:13513–13518
Schmidt KF, Ziu M, Schmidt NO, et al. (2004) Volume reconstruction techniques improve the correlation between histological and in vivo tumor volume measurements in mouse models of human gliomas. J Neuro-oncol 68:207–215
Schmidt NO, Ziu M, Carrabba G, et al. (2004) Antiangiogenic therapy by local intracerebral microinfusion improves treatment efficiency and survival in an orthotopic human glioblastoma model. Clin Cancer Res 10:1255–1262
Sun Y, Schmidt NO, Schmidt K, et al. (2004) Perfusion MRI of U87 brain tumors in a mouse model. Magn Reson Med 51:893–899
Valonen PK, Lehtimaki KK, Vaisanen TH, et al. (2004) Water diffusion in a rat glioma during ganciclovir-thymidine kinase gene therapy-induced programmed cell death in vivo: Correlation with cell density. J Magn Reson Imaging 19:389–396
Vooijs M, Jonkers J, Lyons S, et al. (2002) Noninvasive imaging of spontaneous retinoblastoma pathway-dependent tumors in mice. Cancer Res 62:1862–1867
Jacobs A, Braulich I, Graf R, et al. (2001) Quantitative kinetics of (124I)FIAU in cat and man. J Nucl Med 42:467–475
Voges J, Reszka R, Gossmann A, et al. (2003) Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol 54:479–487
Kircher MF, Mahmood U, King RS, et al. (2003) A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res 63:8122–8125
Macdonald DR, Cascino TL, Schold SC Jr, et al. (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Therasse P, Arbuck SG, Eisenhauer EA, et al. (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Parulekar WR, Eisenhauer EA (2002) Novel endpoints and design of early clinical trials. Ann Oncol 13:139–143
Korn EL, Arbuck SG, Pluda JM, et al. (2001) Clinical trial designs for cytostatic agents: Are new approaches needed? J Clin Oncol 19:265–272
Hanson MW, Hoffman JM, Glantz MJ (1990) FDG-PET in the early postoperative evaluation of patients with brain tumor. J Nucl Med 31:799
Kim EE, Chung SK, Haynie TP, et al. (1992) Differentiation of residual or recurrent tumors from post-treatment changes with F-18 FDG PET. Radiographics 12:269–279
Haberkorn U, Strauss LG, Dimitrakopoulou A, et al. (1993) Fluorodeoxyglucose imaging in advanced head and neck cancer after chemotherapy. J Nucl Med 34:12–17
DiChiro G, Oldfield E, Wright DC, et al. (1988) Cerebral necrosis after radiotherapy and/or intraarterial chemotherapy for brain tumors: PET and neuropathologic studies. AJR Am J Radiol 150:189–197
Ishikawa M, Kikuchi H, Miyatake S, et al. (1993) Glucose consumption in recurrent gliomas. Neurosurgery 33:28–33
DeWitte O, Hildebrand J, Luxen A, et al. (1994) Acute effect of camastine on glucose metabolism in brain and glioblastoma. Cancer 74:2836–2842
Chao ST, Suh JH, Raja S, et al. (2001) The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int J Cancer 96:191–197
Holzer T, Herholz K, Jseke HJ, et al. (1998) FDG-PET as a prognostic indicator in radiochemotherapy of glioblastoma. J Comput Assist Tomogr 17:681–687
Schifter T, Hoffmann JM, Hanson MW, et al. (1993) Serial FDG-PET studies in the prediction of survival in patients with primary brain tumors. J Comput Assist Tomogr 17:509–561
Lilja A, Lundqvist H, Olsson Y, et al. (1989) Positron emission tomography and computed tomography in differential diagnosis between recurrent or residual glioma and treatment-induced brain lesion. Acta Radiol 30:121–128
Segawa H, Fukasawa Y, Miyamato K, et al. (1999) Identification and functional characterization of a Na2+ independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem 274:19745–19751
Woesler B, Kuwert T, Morgenroth C, et al. (1997) Non-invasive grading of primary brain tumours: Results of a comparative study between SPET with 123I-alpha-methyl tyrosine and PET with 18F-deoxyglucose. Eur J Nucl Med 24:428–434
Sonoda Y, Kumabe T, Takahashi T, et al. (1998) Clinical usefulness of 11C-MET PET and 210TI SPECT for differentiation of recurrent glioma from radiation necrosis. Neurol Med Chir 38:342–347
Tsugyuguchi N, Sunada I, Iwai Y, et al. (2003) Methionine positron emission tomography of recurrent metastatic brain tumors and radiation necrosis after stereotactic radiosurgery: Is a differential diagnosis possible? J Neurosurg 98:1056–1064
Dethy S, Goldman S, Belcic S, et al. (1994) Carbon-11-methionine and fluorine-18-FDG-PET study in brain hemeatoma. J Nucl Med 35:1162–1166
Heesters MA, Go KG, Kamman RL, et al. (1998) 11C-tyrosine positron emission tomography and 1H magnetic resonance spectroscopy of the response of brain gliomas to radiotherapy. Neuroradiology 40:103–108
Voges J, Herholz K, Holzer T, et al. (1997) 11C-methionine and 18F-2-fluorodeoxyglucose positron emission tomography: A tool for diagnosis of cerebral glioma and monitoring after brachytherapy with 125I seeds. Stereotact Funct Neurosurg 69:129–135
DiChiro G, De La Paz RL, Brroks RA, et al. (1982) Glucose-utilization of cerebral gliomas measured by (18F) fluorodeoxyglucose and positron emission tomography. Neurology 32:1323–1329
Alavi JB, Alavi A, Chawluk J, et al. (1998) Positron emission tomography in patients with glioma. A predictor of prognosis. Cancer 62:1074–1078
DiChiro G (1987) Positron emission tomography using (18F) fluordeoxyglucose in brain tumors. A powerful diagnostic and prognostic tool. Invest Radiol 22:360–371
Jacobs A, Voges J, Reszka R, et al. (2001) Positron-emission tomography of vector-mediated gene expression in gene therapy for gliomas. Lancet 358:727–729
Muutinen J, Sonninen P, Lehikoinen P, et al. (2000) Radiotherapy treatment planning and long-term follow-up with (11C)methionine PET in patients with low-grade follow-up with (11C)methionione PET in patients with low-grade astrocytoma. Int J Radiat Oncol Biol Phys 48:43–52
Schaller B. State-of-the-art-imaging-methods to investigate the neurovascular mechanism in the origin of Alzheimer’s disease. Differential diagnostic evaluations to other types of dementia (in press)
Aboody K, Brown A, Rainov NG, et al. (2000) Neural stem cells display extensive tropism for pathology in adult brain: Evidence from intracranial gliomas. Proc Natl Acad Sci U S A 97:12846–12851
Benedetti S, Pirola B, Pllo B, et al. (2000) Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med 6:447–450
Schmidt NO, Przylecki W, Yang W, et al. (2005) Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia 7:623–629
Staflin K, Honeth G, Kalliomaki S, et al. (2004) Neural progenitor cell lines rat inhibit tumor growth in vivo. Cancer Res 64:5347–5354
Ethesham M, Kabos P, Kabosova A, et al. (2002) The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res 62:5657–5663
Fomchenko EI, Holland EC (2005) Stem cells and brain cancer. Exp Cell Res 306:323–329
Modo M, Roberts TJ, Sandhu JK, Williams SCR (2004) In vivo monitoring of cellular transplants by magnetic resonance imaging and positron emission tomography. Expert Opin Bio Ther 4:145–155
Zlokovic BV, Apuzzo ML (1997) Cellular and molecular neurosurgery: Pathways from concept to reality—Part 1: Target disorders and concept approaches to gene therapy of the central nervous system. Neurosurgery 40:789–803
Modo M, Hoehn M, Bulte J (2005) Cellular MR imaging. Mol Imaging 4:1–21
Chin BB, Nakamoto Y, Bulte JW, Pittinger MF, Wahl R, Kraitchman DL (2003) 111In oxine labeled mesenchymal stem cell SPET after intravenous administration in myocardial infarction. Nucl Med Commun 24:1149–1154
Shah K, Hsich G, Breakefield XO (2004) Neural precursor cells and their role in neuro-oncology. Dev Neurosci 26:118–130
Koehne G, Doubrovin M, Doubrovina E, et al. (2003) Serial in vivo imaging of targeted migration of human HSV-TK-transduced antigen-specific lymphocytes. Nat Biotechnol 21:405–413
Filmont JE, Czernin J, Yap C, et al. (2003) Value of F-18 fluorodeoxyglucose positron emission tomography for predicting the clinical outcome of patients with aggressive lymphoma prior to and after autologous stem-cell transplantation. Chest 124:608–613
Becherer A, Mitterbauer M, Jaeger U, et al. (2002) Positron emission tomography with [18F]2-fluoro-d-2-deoxyglucose (FDG-PET) predicts relapse of malignant lymphoma after high-dose therapy with stem cell transplantation. Leukemia 16:260–267
Spaepen K, Stroobants S, Dupont P, et al. (2003) Prognostic value of pretransplantation positron emission tomography using fluorine 18-fluorodeoxyglucose in patients with aggressive lymphoma treated with high-dose chemotherapy and stem cell transplantation. Blood 102:53–59
Cremerius U, Fabry U, Wildberger JE, et al. (2002) Pre-transplant positron emission tomography (PET) using fluorine-18-fluoro-deoxyglucose (FDG) predicts outcome in patients treated with high-dose chemotherapy and autologous stem cell transplantation for non-Hodgkin’s lymphoma. Bone Marrow Transplant 30:103–111
Brower V (2005) Search and destroy: Recent research exploits adult stem cells’ attraction to cancer. J Natl Cancer Inst 97:414–416
De Witte O, Lefranc F, Levivier M, et al. (2000) FDG-PET as a prognostic factor in high-grade astrocytoma. J Neuro-oncol 49:157–163
Padma MV, Said S, Jacobs M, et al. (2003) Prediction of pathology and survival by FDG PET in gliomas. J Neuro-oncol 64:227–237
Deshmukh A, Scott JA, Palmer EL, et al. (1996) Impact of fluorodeoxyglucose positron emission tomography on the clinical management of patients with glioma. Clin Nucl Med 21:720–725
Herholz K, Heiss WD (2004) Positron emission tomography in clinical neurology. Mol Imaging Biol 6:239–269
Nariai T, Tanaka Y, Wakimoto H, et al. (2005) Usefulness of L-[methyl-11C] methionine-positron emission tomography as a biological monitoring tool in the treatment of glioma. J Neurosurg 103:498–507
Herholz K, Kracht LW, Heiss WD (2003) Monitoring the effect of chemotherapy in a mixed glioma by C-11-methionine PET. J Neuroimaging 13:269–271
Jacobs AH, Thomas A, Kracht LW, et al. (2005) 18F-fluoro-l-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J Nucl Med 46:1948–1958
Popperl G, Kreth FW, Herms J, et al. (2006) Analysis of 18F-FET PET for grading of recurrent gliomas: Is evaluation of uptake kinetics superior to standard methods? J Nucl Med 47:393–403
Popperl G, Goldbrunner R, Gildehaus FJ, et al. (2005) O-(2-[18F]fluoroethyl)-l-tyrosine PET for monitoring the effects of convection-enhanced delivery of paclitaxel in patients with recurrent glioblastoma. Eur J Nucl Med Mol Imaging 32:1018–1025
Rachinger W, Goetz C, Popperl G, et al. (2005) Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery 57:505–511
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schaller, B.J., Modo, M. & Buchfelder, M. Molecular Imaging of Brain Tumors: A Bridge Between Clinical and Molecular Medicine?. Mol Imaging Biol 9, 60–71 (2007). https://doi.org/10.1007/s11307-006-0069-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-006-0069-9