Abstract
Objective
Fluorophore-labeled contrast imaging agents are moving toward clinical use for a number of applications. The near-infrared dye IRDye 800CW is frequently used in its N-hydroxysuccinamide (NHS) ester form for labeling these agents. Following conjugation or breakdown of a labeled ligand, excess NHS ester is converted to the carboxylate form. To prepare for clinical use as a near-infrared fluorophore, a toxicity study was conducted on IRDye 800CW carboxylate.
Methods
Male and female Sprague–Dawley rats were given a single intravenous or intradermal administration of IRDye 800CW carboxylate; Indocyanine Green was used as a comparative control. Animals were injected with varying doses of the test and control articles and observed for up to 14 days. Clinical chemistry, hematological, and pharmacokinetic analyses were performed on subgroups of animals. Organs were analyzed for content of the test article. Tissues were analyzed microscopically for pathological changes.
Results
Based on hematologic, clinical chemistry, and histopathologic evaluation, single administration of IRDye 800CW carboxylate intravenously at dose levels of 1, 5, and 20 mg/kg or 20 mg/kg intradermally produced no pathological evidence of toxicity.
Conclusion
A dose of 20 mg/kg was identified as the no observed adverse effect level following IV or ID routes of administration of IRDye 800CW.
Similar content being viewed by others
Introduction
With recent advances in optical imaging technology and instrumentation, targeted fluorophore-labeled contrast agents are moving toward translation to human clinical diagnostic use. The primary applications for clinical optical imaging are envisioned to be aids in real-time intraoperative surgical resection of tumors and nodal metastases, endoscopy, and lymphovascular imaging. Near-infrared (NIR) fluorescent dyes (Ex > 750 with red-shifted emission) are the preferred signal-generating molecules for labeling targeted ligands owing to the significant tissue penetration of excitation light and red-shifted emission light and the lack of autofluorescence owing to endogenous fluorochromes [1, 2].
Indocyanine green (ICG) has been used for decades as an angiographic contrast agent and has been recently translated to the clinic for noninvasive lymph mapping [3–6]. More recently, the demonstrated use of ICG at microdose administrations [7, 8] bodes well for NIR fluorescence imaging as a clinically viable molecular imaging modality. Unfortunately, the clinically approved form of ICG does not have a reactive functional group and cannot be used to label targeting agents.
There has been considerable recent effort to develop general-purpose NIR fluorophores that can be conjugated to targeting molecules with the goal of creating targeted contrast agents. IRDye 800CW is a NIR dye that can be functionalized with either an NHS or maleimide reactive group, allowing it to be attached to a number of biomolecules. Tanaka et al. [9] reported the successful use of IRDye 800CW-labeled human serum albumin for sentinel lymph node mapping and surgical resection of spontaneous metastatic melanomas in Sinclair minipigs. In another study, Trastuzumab was dual-labeled with 111In and IRDye 800CW to detect Her2-expressing xenografts in nude mice [10]. The authors concluded that the dual-labeled agent may be an effective agent for tracking Her2 overexpression in breast cancer patients. Houston et al. [11] compared gamma scintigraphy and NIR fluorescence imaging using a dual-labeled cyclic RGD motif to show that on a 1:1 labeling basis, the NIR fluorescence from IRDye 800CW provided greater signal to noise than the radiotracer 111In, even though camera integration times were hundreds of milliseconds rather than minutes long.
Before any agent can be used in human clinical investigations, it must undergo rigorous toxicity testing, the first stage of which must be conducted in animals. In anticipation of its use in humans, we conducted a preliminary study of the toxicity of IRDye 800CW carboxylate since this is the relevant form of the dye remaining after conjugation or breakdown of a labeled ligand. The study was conducted in male and female Sprague–Dawley rats using both IV and ID routes of administration of several dye concentrations. The highest concentration of dye administered was anticipated to be toxic. For comparison, a control set of rats were treated with ICG. To our knowledge, this is the first toxicity report of a NIR dye with the functional potential for labeling targeted ligands. Our study opens new opportunities for safety and toxicity testing of conjugates employing IRDye 800CW.
Materials and Methods
Near-Infrared Dyes
IRDye 800CW carboxylate was obtained from LI-COR Biosciences (Lincoln, NE). The dye structure and purity was confirmed by HPLC, UV/visible spectroscopy, mass spectroscopy, and NMR on multiple dye lots. Purity was >98% by HPLC.
For mass spectroscopy, a 2 μM IRDye 800CW carboxylate solution in 0.1% formic acid was directly infused into an Agilent MSD (SL) electrospray mass spectrometer with ion trap detection.
HPLC analysis was performed on a Polaris C18 3 × 100 mm 3 µm column. Mobile phase: (A) 50 mM triethylammonium acetate buffer (pH 5.8–6.2), 4% acetonitrile and (B) 50 mM triethylammonium acetate buffer (pH 5.8–6.2), 80% acetonitrile. Gradient was 0–100% B over 10 min at 0.5 mL/min.
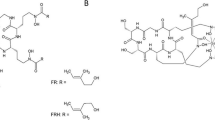
1H NMR was performed via a Bruker AVANCE, 600 MHz, in D2O, TSP-d4. The numbering of the IRDye 800CW protons is shown in Fig. 1a.
Under guidance from the US Food and Drug Administration, it was not necessary to use dye manufactured under GMP for this study; however, it was manufactured by LI-COR under a standard operating protocol as specified by ISO9000-2000 system. ICG, lot no. 81286 (USP lot no. 10B045), was obtained from Akorn, Inc. (Lake Forest, IL). The chemical structures are shown in Fig. 1. The test and control articles were diluted in sterile saline (Baxter Healthcare, Deerfield, IL) and the homogeneity and concentrations of the dosing solutions determined prior to injection.
Safety Study
This study was conducted according to US FDA Good Laboratory Practice regulations 21CFR Part 58 at the GLP facilities at Baylor School of Medicine under the direction of Dr. Marshall. The study animals were assigned to groups as shown in Table 1. To control for bias, animals were stratified by weight in treatment groups. Animals were injected intravenously with either 1, 5, or 20 mg/kg of the test article or intradermally in the dorsal surfaces of both feet with a total dose of 20 mg/kg. Animals were observed twice daily for up to 14 days. A complete necropsy was performed on all animals euthanized during the course of the safety study. Euthanasia was accomplished by exsanguination under isoflurane anesthesia. Blood was collected from safety study animals for clinical chemistry, hematology, and electrolyte analyses. Tissues and organs were processed from all animals euthanized during the safety study and evaluated microscopically after staining with hematoxylin and eosin at HSRL, Inc.
Clinical Pathology
Blood samples were collected while the animals were anesthetized prior to euthanization by exsanguination. Approximately 0.5 mL of blood was collected for hematology and 1.0 mL for serum chemistry whenever possible. Hematology samples were placed in pediatric collection tubes with K2 EDTA (BD Microtainer tubes #365974). Blood samples obtained for serum chemistry were placed in 15-mL conical polypropylene centrifuge tubes. After the samples had clotted, the tubes were centrifuged at 2,500×g for 10 min, and the serum was removed and transferred to a 1.5-mL polypropylene microcentrifuge tube. Normal values for hematology, clinical chemistry, and electrolytes were obtained from several sources [12, 13]
Tissue Distribution
The animal groups for tissue distribution studies are shown in Table 1. IRDye 800CW carboxylate (5 mg/kg) was administered IV via tail vein or ID via the dorsal surface of the feet. Animals of both sexes were exsanguinated while anesthetized 1 h after IV administration and 2 h after ID administration. Organs/tissues collected included brain, liver, kidney, lung, spleen, muscle, reproductive organs, and popliteal lymph nodes. Tissues were weighed, an equal volume of PBS added per gram of tissue, and the tissues homogenized. When possible, 0.5 mL of the homogenate was removed and extracted with 0.75 mL of acetonitrile/methanol (AN/MeOH), 47:3 [14], for each 0.5 mL of homogenate, vortexed for 30 s, and centrifuged for 10 min at 8,000×g. Supernatants were removed, filtered with a 0.45-μm filter (Gelman GHP Acrodisc 13), and placed in autosampler vials. Levels of IRDye 800CW carboxylate present were determined by HPLC (Agilent series 1100 liquid chromatograph) with diode array detection. Because the popliteal lymph nodes and ovaries were too small to homogenize, they were minced with a scalpel prior to extraction with acetonitrile/MeOH.
Pharmacokinetics of IRDye 800CW
For pharmacokinetic analysis, animals were assigned to the groups shown in Table 1. Approximately 0.2 mL of blood was collected for analyses of test and control articles with K2 EDTA as an anticoagulant. Time points utilized for ICG and IRDye 800CW after IV administration included 0, 0.5, 1, 2, 5, 10, 15, 30, 45, and 60 min. Sampling times for ID injection were 0, 5, 10, 30, 60, 90, 120, 180, 240, 360, and 480 min. Blood was collected in pediatric collection tubes with K2EDTA (BD Microtainer tubes #365974). After centrifugation for 10 min at 2,500×g, plasma was removed and extracted with AN/MeOH, 47:3 at a ratio of 1.5 mL AN/MeOH for each milliliter of plasma. After vortexing the samples for 30 s, the samples were centrifuged at 8,000×g for 10 min. The supernatant was removed and filtered through a 0.45-μm filter and placed in autosampler vials for HPLC analysis. Chromatography was performed as indicated below. Analysis of sample results (peak areas) was performed with SigmaPlot 10.0 for regression analysis. WinNonlin Professional 5.2 was used to determine pharmacokinetic parameters by non-compartmental analysis and bolus administration (model 201 for IV administration, model 200 for ID administration).
Chromatography
Dosing solutions, tissue extracts, and plasma extracts were analyzed on an Agilent Model 1100 HPLC at 1 mL/min with a GL Sciences Inertsil ODS-3 column (0.46 × 25 cm) with 3-μm packing material and a model 1100 diode array detector. The mobile phase consisted of 0.05 M triethylammonium acetate, pH 5.5–5.7, filtered through a 0.2-μm Gelman FP-Vericel filter, and acetonitrile. Gradient elution was performed at ambient temperature using predetermined solvent programs. External standards were used to determine plasma and tissue concentrations as well as dosing solution concentrations. Quantification of IRDye 800CW carboxylate and ICG was performed by peak area analysis at 780 nm.
Statistical Analyses
Statistical analyses were performed with SigmaPlot 10.0 (Systat Software, Inc., San Jose, CA) for regression analyses and SPSS 13.0 (SPSS, Inc. Chicago, IL). Typically, linear regression was used to determine the detector response for analytical standards. Means, standard deviations, standard errors, and analysis of variance were calculated with SPSS 13.0. Pharmacokinetic analyses were performed with WinNonlin Professional 5.2 (Pharsight Corp. Cary, NC).
Results
Characterization of IRDye 800CW
UV/vis and Fluorescence (1× PBS) measurements yielded an absorption max. of 774 nm, an emission max. of 789 nm, and an extinction coefficient 240,000. Purity by HPLC at 780 nm was >98%.
Mass spectrometry results were as follows: m/z [M+H+] calc. 1003.2, found 1,003.3, 100%; [M+2H+] calc. 520.1, found 502.1, 45%; [M+Na+], calc. 1,025.3, found 1,025.2, 16%. The MS results are fully consistent with the expected structure of the IRDye 800CW carboxylate dye.
1H NMR results were as follows: (labels refer to Fig. 1a) A/A′ δ 7.71/7.74 (d, J = 1.5 Hz, 2H); C/C′ δ 7.74/7.76 (dd, J = 1.5, 8.1/8.4 Hz, 2H); D/D′ δ 7.21/7.26 (d, J = 8.3/8.4 Hz, 2H); K/K′ δ 7.77/7.82 (d, J = 14/7/14.5 Hz, 2H); J/J′ δ 6.07/6.13 (d, J = 14.3/14.0 Hz, 2H); Q δ 7.25 (d, J = 8.8 Hz, 2H); R δ 7.85 (d, J = 8.8 Hz, 2H); T/Z δ 3.97/3.97 (t, J = 6.9 Hz, 4H); CC δ 2.97 (t, J = 7.2 Hz, 2H); M/M′ δ 2.65 (m, J = 5.7 Hz, 4H); X δ 2.17 (t, J = 7.5 Hz, 2H); N δ 1.96 (m, J = 5.6 Hz, 2H); AA/BB δ 1.85/1.82 (m/m, 4H); U δ 1.73 (tt, J = 7.5 Hz, 2H); W δ 1.59 (tt, J = 7.5 Hz, 2H); V δ 1.36 (tt, J = 7.7 Hz, 2H); I/I′ δ 1.26/1.25 (s, 12H).
The 1H NMR assignments were developed from the 1D, COSY, HSQC, and HMBC spectra using standard pulse sequences for those experiments.
Dosing Solution Analysis
Dosing solutions were analyzed to verify the amount of material delivered to animals (Table 2). ICG was difficult to dissolve in the saline vehicle. The instructions for the preparation of ICG indicated that the lyophilized product should be dissolved in sterile water, which was supplied by the manufacturer. To facilitate solubility, samples were placed in an ultrasonicator and repeatedly forced through a syringe needle to aid solubility prior to administration. The amount of ICG administered varied from 116.9% to 132.4% of the intended dose at 20 mg/kg in the safety study. Animals in the pharmacokinetic study received 95.5% of the intended dose of 5 mg/kg. Homogeneity of the dosing solutions had a coefficient of variation of 2.3–13.4% for the 20 mg/kg solutions and 5.1% for the 5 mg/kg dosing solution.
In contrast, solubility of IRDye 800CW in saline was good even at the 20-mg/kg dose level. The amount and variation in the amount of ICG or IRDye 800CW at the different dose levels is shown in Table 2.
Extraction Efficiency
The method for recovery of dye in plasma and tissue was validated using spiked samples. Saline or plasma was spiked with 50.0 μg/mL of ICG. For IRDye 800CW, plasma was spiked with either 0.5 or 50.0 μg/mL of dye. The percent recovery of ICG from saline was 103.9%, while recovery from plasma was 78.1% based on triplicate analyses. After samples were frozen for 1 day, recovery of ICG was 98.9% for saline and 80.4% from plasma. With IRDye 800CW, plasma recovery was 62.7% and 90.9% at 0.5 and 50 μg/mL, respectively. When samples were frozen for 2 days, recovery was 60.8% at 0.5 μg/mL and 82.0% at 50 μg/mL.
To test recovery from tissue, liver was spiked with IRDye 800CW (100 μg/1.0–1.5 g), homogenized, and extracted. Recovery from liver was 56.9%. Analyses were done in triplicate.
Clinical Observations
Animals were assigned to treatment groups according to weight in order to avoid differences between groups. No statistical significance between initial or final body weights was observed between treatment groups, indicating that no overt toxicity occurred. No toxic effects were observed among any of the animals in this study during routine daily observations. For some animals that received either indocyanine green or IRDye 800CW following intravenous injection, a green color persisted around the injection sites for several days. For animals that received intradermal injections of IRDye 800CW on the dorsal surface of the feet, a green color persisted for several days on all animals. No test or control article-related toxicity was observed at the administration sites following histopathologic evaluation, which correlated with clinical observations.
Tissue Distribution
Tissue distribution was determined in male and female rats given 5 mg/kg of IRDye 800CW. Tissues and plasma were obtained 1 h after IV administration of IRDye 800CW or 2 h after ID administration and processed as described in “Materials and Methods”. Overall means were similar for males and females and between routes of administration for lung, liver, spleen, and muscle (Fig. 2a, b). Slightly higher levels of IRDye 800CW were seen in lung, spleen, and muscle of males compared to females. Liver values were lower after ID administration compared to IV administration. The highest tissue levels were seen in kidney after IV administration, with higher levels found in males compared to females. The reverse was true for ID administration. Levels of IRDye 800CW were greater in ovaries than in testes, and the amount of IRDye 800CW in ovaries was greater in females after ID administration compared to IV administration. No IRDye 800CW was detected in testes 2 h after ID administration. IRDye 800CW was not detected in brain following either IV or ID administration.
Uptake of dye by various organs. a Organ uptake 1 h after IV administration. b Organ uptake 2 h after ID administration. The organ data are average values of six animals. c Popliteal uptake 1 h after IV administration. d Popliteal uptake 2 h after ID administration. Data for popliteal uptake represent individual animals. The upper solid line represents the mean value of IRDye 800CW in males, and the lower solid line represents the mean value in females in both panels.
Uptake of IRDye 800CW in the popliteal lymph nodes varied considerably with the route of administration (Fig. 2c, d). IRDye 800CW levels were much greater after ID administration compared to the IV route. Of all tissues, popliteal uptake was greatest when normalized per gram of tissue after ID administration compared to other organs or tissues.
Pharmacokinetics
In addition to tissue distribution, the pharmacokinetics of plasma levels of both ICG and IRDye 800CW were performed. Pharmacokinetic analysis of IV ICG was performed to serve as a control as published pharmacokinetic values were available for comparison. A single dose level of 5 mg/kg was used for both IV and ID administration. ICG was only administered IV, whereas IRDye 800CW was administered both by the intravenous and intradermal routes. Multiple injections were made in both rear feet for all ID injections in this study.
Pharmacokinetic parameters were determined for IRDye 800CW and ICG using non-compartmental pharmacokinetics. Results of the clearance for IRDye 800CW following IV and ID administration of 5 mg/kg are shown in Fig. 3a, c. The time to peak plasma concentrations was fairly consistent among animals after IV administration, but differed considerably after ID administration. Average values were determined by combining the values obtained for individual animals. The different times to reach peak plasma concentrations after ID administration may be a result of differences in the release of IRDye 800CW. The terminal half-life of ICG determined in this study (Fig. 3b), 6.2 min after IV administration of 5 mg/kg, is similar to that reported in the literature for humans and other species [15, 16]. Clearance half-life of IRDye 800CW following IV injection was determined to be 35.7 min, whereas the half-life following ID injection was 236.5 min. Although urine levels of IRDye 800CW were not evaluated, green-colored urine was observed in the bladders of animals in the tissue distribution and pharmacokinetic studies, which indicates this was a potential route of excretion. Excretion by the kidneys is also indicated by the high tissue levels of IRDye 800CW in kidneys of animals in the tissue distribution study.
Hematology
Blood samples were collected from all animals in the safety study just prior to euthanasia. When samples were clotted or processing was delayed, unreliable values were obtained that were not used to determine group responses. Group averages for hematology data are summarized in Table 3, while data for individual animals are shown in Electronic Supplementary Material (ESM) Fig. 1a–p. After statistical analyses were performed on the data, no clinically significant dose-related changes in hematology were observed 24 h or 14 days after exposure to the test or control articles for either males or females.
For males, all parameters analyzed were generally within the normal ranges for the respective tests (Table 3; ESM Fig. 1a–p). A slight elevation was noted in the hematocrit and hemoglobin of control males and high-dose IV IRDye 800CW groups, but were deemed insignificant. While slight differences were noted between some of the values for day 15 as compared to day 2, the values fell within the normal ranges. There was no evidence of a dose or route of administration-related response.
Values were similar for males and females with the exception of white blood cells, which were slightly lower in females. There was no evidence of a dose-related difference between intravenous or intradermal administration.
Liver and Kidney Function
For males analyzed 24 h post-injection, all parameters were within the normal range for the respective tests (Table 4; ESM Fig. 2a–m), with the exception of LDH and creatine kinase that were elevated outside the normal range in animals that received the vehicle control of intravenous saline. Alkaline phosphatase was elevated above the normal range in all animals regardless of treatment.
At 15 days post-injection, all parameters analyzed for male liver function were within the normal ranges for the respective tests (Table 4; ESM Fig. 2a–m), with the exception of GGT in the mid-dose IRDye 800CW group and creatinine kinase in the high-dose IV IRDye 800CW group that were elevated outside the normal range. Examination of the data for individual animals (ESM Fig. 2c, f) indicated that the averages for these values were skewed by a single animal. The average value for LDH (Table 4; ESM Fig 2d) was similarly skewed in the ID IRDye 800CW group by two animals. All of the individual skewed values were from different animals. None of these animals had more than a single value outside of the normal range. Globulin was also elevated in the mid-dose IRDye 800CW group, and total bilirubin was elevated in the ICG group. As with the 24-h groups, alkaline phosphatase levels were elevated above the normal range in all animals regardless of treatment, except for the animals in the ID IRDye 800CW group which was in the normal range. There was no evidence of a dose-related response or from the two different routes of administration. Overall, hepatic function test results were similar in the 14-day and 24-h treatment animals. The elevated values described above did not have any clinical significance for the treatment groups 15 days after exposure to the test or control article.
Similarly, females showed no evidence of a dose-related response or differences from the two different routes of administration 24 h post-injection (Table 4; ESM Fig. 2a–m). Values obtained from females were similar to those seen in males, with the exception of ALT and alkaline phosphatase which were lower in females than in males. As seen in males, LDH and creatinine kinase were elevated outside the normal range in animals that received the saline vehicle control intravenously. LDH was also elevated above the high normal range in animals that received ICG. ALT values were below normal in animals that received the 5- and 20-mg/kg doses of IRDye 800CW, but no clinical significance could be attributed to this observation.
Again, at 15 days post-injection, there was no evidence of a dose-related response or differences from the two different routes of administration for liver function in females. Values obtained from females were similar to those seen in males, with the exception of alkaline phosphatase which was not elevated in females compared to males. As seen in males, LDH was slightly elevated outside the normal range in females that received the saline vehicle control intravenously. LDH was also slightly elevated after 14 days in the vehicle control, ICG, and low-dose IRDye 800CW groups. Creatine kinase was elevated in the vehicle control group. Globulin levels were also elevated above the high normal range in animals that received mid and high doses of IRDye 800CW. ALT values were below normal in animals that received the 5- and 20-mg/kg doses of IRDye 800CW, but no clinical significance could be attributed to this observation. Results from females 14 days after treatment were similar to those from females in the 24-h treatment group.
Electrolytes
At 24 h post-injection, all parameters analyzed were within the normal ranges for the respective tests for males and females (Table 5). There was no evidence of either a dose or route of administration-related response. Although potassium and phosphorous levels were lower in females than males, the difference was not clinically significant.
Fifteen days post-injection, all parameters analyzed were within the normal ranges for the respective tests (Table 5) for males and females, with the exception of the male mid-dose IRDye 800CW group potassium levels in which the mean was slightly lower than the low normal value. There was no evidence of either a dose or route of administration-related response.
Necropsy
Gross necropsies were conducted on all animals in the safety study, including extra animals in the high-dose groups. Because no lesions were observed in the extra animals, no tissues were submitted for histopathologic evaluation. Some animals in the highest group appeared to have excess red fluid (presumably blood) around the brain, so additional rats were included in the day 2 female high-dose groups, which were dosed separately from the day 2 males. No consistent findings were observed to verify the observations in males, and there was no confirmation of adverse findings after histopathologic evaluation of the brains of males or females in the day 2 or day 15 groups, nor was this seen in animals in the tissue distribution study that had 1- or 2-h exposures to the test article.
Organ Weights
Organs were weighed at necropsy to determine any gross effects of the test or control article on specific organ systems. No statistically significant differences were observed between groups when absolute organ weights were compared or when relative organ weights were compared. One male rat in the day 15 ID IRDye 800CW group had an enlarged spleen. On histopathologic evaluation of the spleen from this animal, increased hemopoiesis (grade 1) was noted. As grade 1 hemopoiesis was also seen in animals in the vehicle control group and animals in other groups that received test article by IV administration, the observation of an enlarged spleen in this animal was deemed to be unrelated to test article administration.
Discussion
This study was aimed at examining the toxicity of the NIR dye IRDye 800CW for use in conjugates administered for NIR fluorescence molecular imaging in humans. To our knowledge, this is the first study of the toxicity of a NIR dye with the potential for functionalization and labeling of biomolecules. Fluorescein (excitation 494 nm, emission 518 nm) has been cleared by the United States Food and Drug Administration as a contrast agent for angiography and has been extensively used for the detection of choroidal neovascularization associated with macular degeneration and for intraoperative coronary angiography [17–21]. However, fluorescein’s excitation and emission wavelengths are in a region where significant tissue autofluorescence exists, limiting its usefulness.
The NIR dye ICG was approved by the FDA in 1958 [22] as a contrast agent for retinal angiography. Since then, it has been used extensively for angiographic analysis of a variety of ocular pathologies [22]. In animals, a number of deleterious effects have been reported following retinal injection and prolonged exposure of the retinal pigment epithelium to ICG, including cellular atrophy and the induction of apoptosis [23–27]. In these studies, however, the dose of ICG was significantly above the clinical dose in humans [23–27].
At the recommended ICG clinical dose (approximately 3.5 μg/kg) [25], human studies have reported adverse reactions that include pain at the injection site, low blood pressure, and itching [28, 29]. Fatal anaphylactic reactions have been reported in two cases [28, 29]. However, the estimated death rate due to administration of ICG is extremely low [28, 29].
As noted above, the form of ICG approved for clinical applications does not contain a reactive functional group precluding its use as a general-purpose label for targeted NIR contrast agents. Prior to human administration, an NIR contrast agent must undergo a three-phase test in animals consisting of an examination of the toxicity of the dye, the targeting moiety, and the final conjugate owing to the fact that linking the targeting ligand and fluorophore results in a new biomolecule that may have different properties.
In this study, we have examined the toxicity of IRDye 800CW at elevated levels with the intent of using it as a signaling molecule for conjugation to ligands targeting a variety of cellular biomolecules. Following histopathologic evaluation of tissues, no systemic or local toxicity was observed either 1 day or 14 days after either IV or ID injection of either ICG or IRDye 800CW at doses up to 20 mg/kg. Minimal perivascular hemorrhage at the injection site was seen in a few animals that received tail vein injections. This change was sometimes accompanied by low-grade inflammation and was considered to be the result of venipuncture trauma. Based on hematologic and clinical chemistry evaluations as well as the histopathologic examination, a single administration of IRDye 800CW IV at levels of 1, 5, and 20 mg/kg or 20 mg/kg ID followed by 14 days of observation produced no evidence of pathological effect or toxicity. The 20-mg/kg dose was identified as the no observed adverse effect level (NOAEL) for IRDye 800CW administered either IV or ID. The NOAEL for ICG was also 20 mg/kg in this study. This is equivalent to a dose of 1.36 g of dye injected per 68 kg individual, which is approximately 10,000 times more than the projected dose at which IRDye 800CW would be used.
Instrumentation capable of visualizing targeted agents labeled with IRDye 800CW are in existence, and several have regulatory clearance. The Zeiss Pentero (Carl Zeiss GmbH) and the Leica FL800 (Leica Microsystems, USA) have been used with ICG for surgical resectioning of aneurysms and are predicted to be adaptable to intraoperative surgical resection of tumors using NIR-labeled targeted agents. The fluorescence-assisted resection and exploration instrumentation system described by Tanaka [10], the optical system described by Sevick-Muraca and coworkers [8, 9], and the Artemis (O2View, Marken, the Netherlands) also have great potential for NIR fluorescence molecular imaging.
A recent study [30] described the use of a novel functionalized ICG to label Cetuximab in order to define surgical margins; however, the authors concluded that ICG lacked the sensitivity needed for use in a clinical setting. Given that IRDye 800CW is >50 times brighter than ICG [9], it may be especially suited for molecular imaging of disease markers at picomolar to femtomolar tissue concentrations.
In parallel with this study, we are finalizing the filing of a drug master file (DMF) with the US Food and Drug Administration. The data from the completed toxicity study combined with the DMF will enable us to participate in the filing of several planned investigational new drug applications for new contrast imaging agents for intraoperative surgery and lymphatic analysis. IRDye 800CW dye made under GMP compliance will be available for these studies by the first part of 2010. While more work remains to establish the safety and efficacy of IRDye 800CW-labeled conjugates, the lack of pathological effects of IRDye 800CW reported here is promising. With the current environment of NIR fluorescence instrument development, IRDye 800CW should impact molecular imaging by providing a functionalized NIR dye, enabling a variety of new targeting contrast agents.
References
Gurfinkel M, Ke S, Wen X, Li C, Sevick-Muraca EM (2005) Near-infrared fluorescence optical imaging and tomography. Dis Markers 19:107–121
Adams KE, Ke S, Kwon S et al. (2007) Comparison of visible and near-infrared wavelength excitable fluorescent dyes for molecular imaging. J Biomed Optics 12:024017. doi:12:024017-1-024017-9
Kitai T, Inomoto T, Miwa M, Shikayama T (2005) Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer 12:211–215
Unno N, Inuzuka K, Suzuki M et al. (2007) Preliminary evidence with a novel fluorescence lymphography using indocyanine green fluorescence lymphography. J Vasc Surg 45:1016–1021
Tagaya N, Yamazaki R, Nakagawa A et al. (2008) Intraoperative identification of sentinel lymph nodes by near-infrared fluorescence imaging in patients with breast cancer. Am J Surg 195:850–853
Unno N, Nishiyama M, Suzuki M et al. (2008) Quantitative lymph imaging for assessment of lymph function using indocyanine green fluorescence lymphography. Eur J Vasc Endovasc Surg 36:230–236
Sevick-Muraca EM, Sharma R, Rasmussen JC et al. (2008) Imaging of lymph flow in breast cancer patients following microdose administration of a near-infrared fluorophore: feasibility study. Radiology 246:734–741
Rasmussen JC, Tan I-C, Marshall MV, Fife CE, Sevick-Muraca EM (2009) Lymphatic imaging in humans with near infrared fluorescence. Curr Opin Biotechnol 20:1–9
Tanaka E, Choi HS, Fujii H, Bawendi MG, Frangioni JV (2006) Image-guided oncologic surgery using invisible light: completed pre-clinical development for sentinel lymph node mapping. Ann Surg Oncol 13:1671–1681
Sampath L, Kwon S, Ke S, Wang W, Schiff R, Mawad ME, Sevick-Muraca EM (2007) Dual-labeled trastuzumab-based imaging agent for the detection of human epidermal growth factor receptor 2 overexpression in breast cancer. J Nucl Med 48:1501–1510
Houston JP, Ke S, Wang W, Li C, Sevick-Muraca EM (2005) Quality analysis of in vivo near-infrared fluorescence and conventional gamma images acquired using a dual-labeled tumor-targeting probe. J Biomed Optics 10:054010
Lillie LE, Temple NJ, Florence LZ (1996) Reference values for young normal Sprague-Dawley rats: weight gain, hematology, and clinical chemistry. Human and Exp Toxicol 15:612–616
Giknis MLA, Clifford CB (2006) Clinical Laboratory parameters for Crl:CD(SD) rats. Charles River Laboratories, pp 1–14
Ott P, Keiding S, Bass L (1993) Plasma elimination of indocyanine green in the intact pig after bolus injection and during constant infusion: comparison of spectrophotometry and high-pressure liquid chromatography for concentration analysis. Hepatology 18:1504–1515
Bax NDS, Tucker GT, Woods HF (1980) Lignocaine and indocyanine green kinetics in patients following myocardial infarction. Br J Pharmacol 10:353–362
Klaassen CD, Plaa GL (1969) Plasma disappearance and biliary excretion of indocyanine green in rats, rabbits, and dogs. Tox Appl Pharmacol 15:374–384
Shah SM, Tatlpinar S, Quinlan E et al. (2006) Dynamic and quantitative analysis of choroidal neovascularization by fluorescein angiography. Invest Ophthalmol Vis Sci 47:5460–5468
Sykes SO, Bressler NM, Maguire MG, Schachat AP, Bressler SB (1994) Detecting recurrent choroidal neovascularization: comparison of clinical examination with and without fluorescein angiography. Arch Ophthalmol 112:1561–1566
Takayama T, Wanibuchi Y, Suma H et al. (1991) Intraoperative coronary angiography using fluorescein. Ann Thorac Surg. 51:140–143
Takayama T, Wanibuchi Y, Suma H et al. (1992) Intraoperative coronary angiography using fluorescein: basic studies and clinical application. Vascular and Endovascular Surg 26:193–199
Yannuzzi LA, Flower RW, Slakter JS (1997) Indocyanine green angiography. Mosby Press, St. Louis
Agarwal A (2007) Fundus fluorescein and indocyanine green angiography: a textbook and atlas. Slack Inc., Thorofare
Ikagawa H, Yoneda M, Iwaki M et al. (2005) Chemical toxicity of indocyanine green damages retinal pigment epithelium. Invest Ophthalmol Vis Sci 46:2531–2539
Lüke C, Lüke M, Dietlein TS et al. (2005) Retinal tolerance to dyes. Br J Ophthalmol 89:1188–1191
Schuettauf F, Haritoglou C, May CA et al. (2006) Administration of novel dyes for intraocular surgery: an in vivo toxicity animal study. Invest Ophthalmol Vis Sci 47:3573–3578
Gandorfer A., Haritoglou C., Gandorfer A et al. (2003) Retinal damage from indocyanine green in experimental macular surgery. Invest Ophthalmol Vis Sci 44:316–323
Hope-Ross M, Yannuzzi LA, Gragoudas ES et al. (1994) Adverse reactions due to indocyanine green. Ophthalmology 101:529–533
Obana A, Miki M, Havashi K et al. (1994) Survey of complications of indocyanine green angiography in Japan. Am J Ophthalmol 118:749–753
Raabe A, Nakaji P, Beck J et al. (2005) Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J Neurosurg 103:982–989
Withrow KP, Gleysteen BS, Safavy A, Skipper J, Desmond RA, Zinn K, Rosenthal EL (2007) Assessment of indocyanine green-labeled cetuximab to detect xenografted head and neck cancer cell lines. Otolaryngol-Head Neck Surg 137:729–734
Disclosure Statement
Authors M.V.M. and E.M.S.-M. have no conflicts. Authors D.D. and D.M.O. are employed by LI-COR Biosciences.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Supplemental Fig. 1
Variability of hematological values. Normal ranges are demarcated by red dotted lines. The average value for each group is shown by the green line. (RTF 1691 kb)
Supplemental Fig. 2
Variability of clinical chemistry values. Normal ranges are demarcated by red dotted lines. The average value for each group is shown by the green line. (RTF 1434 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Marshall, M.V., Draney, D., Sevick-Muraca, E.M. et al. Single-Dose Intravenous Toxicity Study of IRDye 800CW in Sprague-Dawley Rats. Mol Imaging Biol 12, 583–594 (2010). https://doi.org/10.1007/s11307-010-0317-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-010-0317-x