Abstract
Purpose
The aim of this study was to understand the relationship of lipid deposition to the macrophage content, macrophage metabolism, and apoptosis in plaque. We compared the uptake of 2-deoxy-2-fluoro-D-[14C]glucose ([14C]FDG) and [99mTc]HYNIC-annexin V ([99mTc]annexin A5) with the lesion histology in apolipoprotein E knockout (apoE−/−) mice.
Procedures
Male apoE−/− mice (n = 9) were injected with [14C]FDG and [99mTc]annexin A5. Cryostat sections of aorta samples (n = 49) were used for dual-tracer autoradiography, and regional tracer uptake levels were evaluated. Lesions were identified histologically with Movat's pentachrome (AHA lesion phenotypes), Mac-2 staining (macrophage infiltration) and Oil Red O staining (lipid deposition).
Results
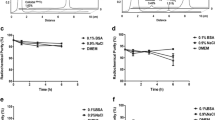
The highest uptakes of [14C]FDG (3.10 ± 1.50 %ID × kilogram per square millimeter) and [99mTc]annexin A5 (0.49 ± 0.20 %ID × kilogram per square millimeter) were shown in atheromatous lesions (types III and IV). Each tracer uptake showed better correlation with macrophage infiltration than lipid deposition ([14C]FDG, r = 0.44 vs. r = 0.14; [99mTc]annexin A5, r = 0.65 vs. r = 0.48).
Conclusions
Both tracers were concentrated in type III and IV atheromatous lesions which corresponded to macrophage infiltration rather than lipid deposition.




Similar content being viewed by others
Abbreviations
- FDG:
-
2-Deoxy-2-fluoro-D-glucose
- HYNIC:
-
Hydrazinonicotinamide
- [99mTc]annexin A5:
-
[99mTc]HYNIC-annexin A5
- apoE−/− mice:
-
Apolipoprotein E knockout mice
- PET:
-
Positron emission tomography
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance image
- TIA:
-
Transient ischemic attack
- ARG:
-
Autoradiography
- ROI:
-
Region of interest
- ACS:
-
Acute coronary syndrome
References
Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM (2000) Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 20:1262–1275
Virmani R, Burke AP, Willerson JT, Farb A, Narula J, Kolodgie FD (2007) The pathology of vulnerable plaque. In: The Vulnerable Atherosclerotic Plaque: Strategies for Diagnosis and Management. Chapter 2, 21–36
Bartorelli AL, Potkin BN, Almagor Y, Keren G, Roberts WC, Leon MB (1990) Plaque characterization of atherosclerotic coronary arteries by intravascular ultrasound. Echocardiography 7:389–395
Thieme T, Wernecke KD, Meyer R et al (1996) Angioscopic evaluation of atherosclerotic plaques: validation by histomorphologic analysis and association with stable and unstable coronary syndromes. J Am Coll Cardiol 28:1–6
Cury RC, Houser SL, Furie KL et al (2006) Vulnerable plaque detection by 3.0 tesla magnetic resonance imaging. Invest Radiol 41:112–115
Estes JM, Quist WC, Lo Gerfo FW, Costello P (1998) Noninvasive characterization of plaque morphology using helical computed tomography. J Cardiovasc Surg (Torino) 39:527–534
Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352:1685–1695
Stoneman VE, Bennett MR (2004) Role of apoptosis in atherosclerosis and its therapeutic implications. Clin Sci (Lond) 107:343–354
Davies JR, Rudd JH, Weissberg PL, Narula J (2006) Radionuclide imaging for the detection of inflammation in vulnerable plaques. J Am Coll Cardiol 47(8 Suppl):C57–C68
Laufer EM, Winkens MH, Narula J, Hofstra L (2009) Molecular imaging of macrophage cell death for the assessment of plaque vulnerability. Arterioscler Thromb Vasc Biol 29:1031–1038
Ogawa M, Magata Y, Kato T et al (2006) Application of 18F-FDG PET for monitoring the therapeutic effect of antiinflammatory drugs on stabilization of vulnerable atherosclerotic plaques. J Nucl Med 47:1845–1850
Rudd JH, Myers KS, Bansilal S et al (2007) (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol 50:892–896
Ishino S, Kuge Y, Takai N et al (2007) 99mTc-annexin A5 for noninvasive characterization of atherosclerotic lesions: imaging and histological studies in myocardial infarction-prone Watanabe heritable hyperlipidemic rabbits. Eur J Nucl Med Mol Imaging 34:889–899
Kolodgie FD, Petrov A, Virmani R et al (2003) Targeting of apoptotic macrophages and experimental atheroma with radiolabeled annexin V: a technique with potential for noninvasive imaging of vulnerable plaque. Circulation 108:3134–3139
Kietselaer BL, Reutelingsperger CP, Heidendal GA et al (2004) Noninvasive detection of plaque instability with use of radiolabeled annexin A5 in patients with carotid-artery atherosclerosis. N Engl J Med 350:1472–1473
Tahara N, Kai H, Nakaura H et al (2007) The prevalence of inflammation in carotid atherosclerosis: analysis with fluorodeoxyglucose-positron emission tomography. Eur Heart J 28:2243–2248
Ben-Haim S, Kupzov E, Tamir A, Israel O (2004) Evaluation of 18F-FDG uptake and arterial wall calcifications using 18F-FDG PET/CT. J Nucl Med 45:1816–1821
Arauz A, Hoyos L, Zenteno M, Mendoza R, Alexanderson E (2007) Carotid plaque inflammation detected by 18F-fluorodeoxyglucose-positron emission tomography. Pilot study. Clin Neurol Neurosurg 109:409–12
Davies JR, Rudd JH, Fryer TD et al (2005) Identification of culprit lesions after transient ischemic attack by combined 18F fluorodeoxyglucose positron-emission tomography and high-resolution magnetic resonance imaging. Stroke 36:2642–2647
Blankenberg FG, Katsikis PD, Tait JF et al (1998) In vivo detection and imaging of phosphatidylserine expression during programmed cell death. Proc Natl Acad Sci USA 95:6349–6354
Zhao Y, Kuge Y, Zhao S, Morita K, Inubushi M, Strauss HW, Blankenberg FG, Tamaki N (2007) Comparison of 99mTc-annexin A5 with 18F-FDG for the detection of atherosclerosis in ApoE−/− mice. Eur J Nucl Med Mol Imaging 34:1747–1755
Kasprzak KS, Dencker L, Larsson BS et al (1991) Isotopic and nuclear analytical techniques in biological systems: a critical survey. Pure Appl Chem 63:1269–1306
Brown RS, Leung JY, Fisher SJ, Frey KA, Ethier SP, Wahl RL (1995) Intratumoral distribution of tritiated fluorodeoxyglucose in breast carcinoma. I. Are inflammatory cells important? J Nucl Med 36:1854–1861
Movat HZ (1955) Demonstration of all connective tissue elements in a single section; pentachrome stains. AMA Arch Pathol 60:289–295
Kowala MC, Recce R, Beyer S, Gu C, Valentine M (2000) Characterization of atherosclerosis in LDL receptor knockout mice: macrophage accumulation correlates with rapid and sustained expression of aortic MCP-1/JE. Atherosclerosis 149:323–330
Stary HC, Chandler AB, Glagov S et al (1994) A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, American heart association. Circulation 89:2462–2478
Stary HC, Chandler AB, Dinsmore RE et al (1995) A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, American heart association. Circulation 92:1355–1374
Ni M, Chen WQ, Zhang Y (2009) Animal models and potential mechanisms of plaque destabilisation and disruption. Heart 95:1393–1398
Jackson CL, Bennett MR, Biessen EA, Johnson JL, Krams R (2007) Assessment of unstable atherosclerosis in mice. Arterioscler Thromb Vasc Biol 27:714–720
Kockx MM, De Meyer GR, Muhring J, Jacob W, Bult H, Herman AG (1998) Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation 97:2307–2315
Lutgens E, de Muinck ED, Kitslaar PJ, Tordoir JH, Wellens HJ, Daemen MJ (1999) Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc Res 41:473–479
Geng YJ, Phillips JE, Mason RP, Casscells SW (2003) Cholesterol crystallization and macrophage apoptosis: implication for atherosclerotic plaque instability and rupture. Biochem Pharmacol 66:1485–1492
Rogers IS, Nasir K, Figueroa AL et al (2010) Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovasc Imaging 3:388–397
Silvera SS, Aidi HE, Rudd JH et al (2009) Multimodality imaging of atherosclerotic plaque activity and composition using FDG-PET/CT and MRI in carotid and femoral arteries. Atherosclerosis 207:139–143
Laitinen I, Marjamäki P, Haaparanta M et al (2006) Non-specific binding of [18F]FDG to calcifications in atherosclerotic plaques: experimental study of mouse and human arteries. Eur J Nucl Med Mol Imaging 33:1461–1467
Rudd JH, Myers KS, Bansilal S et al (2009) Relationships among regional arterial inflammation, calcification, risk factors, and biomarkers: a prospective fluorodeoxyglucose positron-emission tomography/computed tomography imaging study. Circ Cardiovasc Imaging 2:107–115
Fayad ZA, Fuster V (2001) Clinical imaging of the high-risk or vulnerable atherosclerotic plaque. Circ Res 89:305–316
Mollet NR, Cademartiri F, Nieman K et al (2004) Multislice spiral computed tomography coronary angiography in patients with stable angina pectoris. J Am Coll Cardiol 43:2265–2270
Wilensky RL, Song HK, Ferrari VA (2006) Role of magnetic resonance and intravascular magnetic resonance in the detection of vulnerable plaques. J Am Coll Cardiol 47(8 Suppl):C48–56
Kooi ME, Cappendijk VC, Cleutjens KB et al (2003) Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation 107:2453–2458
Nissen SE, Yock P (2001) Intravascular ultrasound: novel pathophysiological insights and current clinical applications. Circulation 103:604–616
Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J (1993) Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J 69:377–381
Kolodgie FD, Burke AP, Farb A et al (2001) The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardio 16:285–292
Johnson LL, Schofield L, Donahay T, Narula N, Narula J (2005) 99mTc-annexin V imaging for in vivo detection of atherosclerotic lesions in porcine coronary arteries. J Nucl Med 46:1186–1193
Isobe S, Tsimikas S, Zhou J et al (2006) Noninvasive imaging of atherosclerotic lesions in apolipoprotein E-deficient and low-density-lipoprotein receptor-deficient mice with annexin A5. J Nucl Med 47:1497–1505
Bessell EM, Foster AB, Westwood JH (1972) The use of deoxyfluoro-D-glucopyranoses and related compounds in a study of yeast hexokinase specificity. Biochem J 128:199–204
Nibbering PH, Leijh PC, Van Furth R (1987) Quantitative immunocytochemical characterization of mononuclear phagocytes. I. Monoblasts, promonocytes, monocytes, and peritoneal and alveolar macrophages. Cell Immunol 105:374–385
Nibbering PH, Leijh PC, Van Furth R (1987) Quantitative immunocytochemical characterization of mononuclear phagocytes. II. Monocytes and tissue macrophages. Immunology 62:171–176
Bjorkerud S, Bjorkerud B (1996) Apoptosis is abundant in human atherosclerotic lesions, especially in inflammatory cells (macrophages and T cells), and may contribute to the accumulation of gruel and plaque instability. Am J Pathol 149:367–380
Acknowledgments
This study was partially supported by Special Coordination Funds for Promoting Science and Technology of the Ministry of Education, Culture, Sports, Science and Technology, the Japanese government. This research was also partially supported by a Grant-in-Aid for General Scientific Research from the Japan Society for the Promotion of Science. The authors would like to thank the staff of the Department of Nuclear Medicine and Central Institute of Isotope Science, Hokkaido University and the Facility of Radiology, Hokkaido University Medical Hospital for supporting this work. We also thank the NCI-Frederick Cancer Research and Development Center for providing annexin A5.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Significance:
In this study we compared the lesion distribution of [99mTc]annexin A5 (marker of ongoing apoptosis) and [14C]FDG (marker of the increased metabolism of inflammatory cells) with lesion histology in atherosclerotic plaques of apoE−/− mice. This data clarified the pathophysiologic significance of molecular imaging using [99mTc]annexin A5 and [14C]FDG, indicated the potential of the two probes on unstable plaque detection, and suggested the reason why discrepancy may be observed between [18F]FDG imaging and other diagnostic imaging or clinical outcomes.
Rights and permissions
About this article
Cite this article
Zhao, Y., Zhao, S., Kuge, Y. et al. Localization of Deoxyglucose and Annexin A5 in Experimental Atheroma Correlates with Macrophage Infiltration but not Lipid Deposition in the Lesion. Mol Imaging Biol 13, 712–720 (2011). https://doi.org/10.1007/s11307-010-0389-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-010-0389-7