Abstract
Purpose
This study aims to evaluate 64Cu-DOTA-rituximab (PETRIT) in a preclinical transgenic mouse model expressing human CD20 for potential clinical translation.
Procedures
64Cu was chelated to DOTA-rituximab. Multiple radiolabeling, quality assurance, and imaging experiments were performed. The human CD20 antigen was expressed in B cells of transgenic mice (CD20TM). The mice groups studied were: (a) control (nude mice, n = 3) that received 7.4 MBq/dose, (b) with pre-dose (CD20TM, n = 6) received 2 mg/kg pre-dose of cold rituximab prior to PETRIT of 7.4 MBq/dose, and (c) without pre-dose (CD20TM, n = 6) PETRIT alone received 7.4 MBq/dose. Small animal PET was used to image mice at various time points (0, 1, 2, 4, 24, 48, and 72 h). The OLINDA/EXM software was used to determine the human equivalent dose for individual organs.
Results
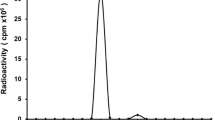
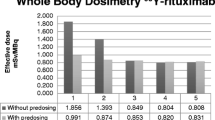
PETRIT was obtained with a specific activity of 545 ± 38.91 MBq/nmole, radiochemical purity >95%, and immunoreactivity >75%. At 24 h, spleenic uptake of PETRIT%ID/g (mean ± STD) with and without pre-dose was 1.76 ± 0.43% and 16.5 ± 0.45%, respectively (P value = 0.01). Liver uptake with and without pre-dose was 0.41 ± 0.51% and 0.52 ± 0.17% (P value = 0.86), respectively. The human equivalents of highest dose organs with and without pre-dose are osteogenic cells at 30.8 ± 0.4 μSv/MBq and the spleen at 99 ± 4 μSv/MBq, respectively.
Conclusions
PET imaging with PETRIT in huCD20 transgenic mice provided human dosimetry data for eventual applications in non-Hodgkins lymphoma patients.




Similar content being viewed by others
References
Juweid ME, Cheson BD (2006) Positron-emission tomography and assessment of cancer therapy. N Engl J Med 354:496–507
Larson SM (1985) Radiolabeled monoclonal anti-tumor antibodies in diagnosis and therapy. J Nucl Med 26:538–545
Wu AM, Yazaki PJ, Tsai S, Nguyen K, Anderson AL, McCarthy DW et al (2000) High-resolution microPET imaging of carcinoembryonic antigen-positive xenografts by using a copper-64-labeled engineered antibody fragment. Proc Natl Acad Sci U S A 97:8495–8500
Goldenberg DM, DeLand F, Kim E, Bennett S, Primus FJ, van Nagell JR et al (1978) Use of radio-labeled antibodies to carcinoembryonic antigen for the detection and localization of diverse cancers by external photoscanning. N Engl J Med 298:1384–1388
Adams GP, Schier R, McCall AM, Crawford RS, Wolf EJ, Weiner LM et al (1998) Prolonged in vivo tumour retention of a human diabody targeting the extracellular domain of human HER2/neu. Br J Cancer 77:1405–1412
Cheson BD, Leonard JP (2008) Monoclonal antibody therapy for B-cell non-Hodgkin's lymphoma. N Engl J Med 359:613–626
Dearling JL, Voss SD, Dunning P, Snay E, Fahey F, Smith SV, et al (2011) Imaging cancer using PET—the effect of the bifunctional chelator on the biodistribution of a (64)Cu-labeled antibody. Nucl Med Biol 38: 29–38
Forrer F, Chen J, Fani M, Powell P, Lohri A, Muller-Brand J et al (2009) In vitro characterization of (177)Lu-radiolabelled chimeric anti-CD20 monoclonal antibody and a preliminary dosimetry study. Eur J Nucl Med Mol Imaging 36:1443–1452
Zhou X, Hu W, Qin X (2008) The role of complement in the mechanism of action of rituximab for B-cell lymphoma: implications for therapy. Oncologist 13:954–966
Glennie MJ, French RR, Cragg MS, Taylor RP (2007) Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol 44:3823–3837
Chappell LL, Dadachova E, Milenic DE, Garmestani K, Wu C, Brechbiel MW (2000) Synthesis, characterization, and evaluation of a novel bifunctional chelating agent for the lead isotopes 203Pb and 212Pb. Nucl Med Biol 27:93–100
Chappell LL, Ma D, Milenic DE, Garmestani K, Venditto V, Beitzel MP et al (2003) Synthesis and evaluation of novel bifunctional chelating agents based on 1,4,7,10-tetraazacyclododecane-N, N′, N″, N″′-tetraacetic acid for radiolabeling proteins. Nucl Med Biol 30:581–595
Li J, Zheng H, Trent J, Bates P, Ng CK (2009) Evaluation of 64Cu-DOTA-AS1411 as a PET tracer for lung cancer imaging. J Nucl Med Meet (Abstracts) 50:1915
Li L, Bading J, Yazaki PJ, Ahuja AH, Crow D, Colcher D et al (2007) A versatile bifunctional chelate for radiolabeling humanized anti-CEA antibody with In-111 and Cu-64 at either thiol or amino groups: PET imaging Of CEA-positive tumors with whole antibodies. Bioconjugate Chem 19:89–96
Lu SX, Takach EJ, Solomon M, Zhu Q, Law SJ, Hsieh FY (2005) Mass spectral analyses of labile DOTA-NHS and heterogeneity determination of DOTA or DM1 conjugated anti-PSMA antibody for prostate cancer therapy. J Pharm Sci 94:788–797
Grunberg J, Novak-Hofer I, Honer M, Zimmermann K, Knogler K, Blauenstein P et al (2005) In vivo evaluation of 177Lu- and 67/64Cu-labeled recombinant fragments of antibody chCE7 for radioimmunotherapy and PET imaging of L1-CAM-positive tumors. Clin Cancer Res 11:5112–5120
Zimmermann K, Grünberg J, Honer M, Ametamey S, August Schubiger P, Novak-Hofer I (2003) Targeting of renal carcinoma with 67/64Cu-labeled anti-L1-CAM antibody chCE7: selection of copper ligands and PET imaging. Nucl Med Biol 30:417–427
Novak-Hofer I, Zimmermann K, Maecke HR, Amstutz HP, Carrel F, Schubiger PA (1997) Tumor uptake and metabolism of copper-67-labeled monoclonal antibody chCE7 in nude mice bearing neuroblastoma xenografts. J Nucl Med 38:536–544
Lindmo T, Boven E, Cuttitta F, Fedorko J, Bunn PA Jr (1984) Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods 72:77–89
Saludes JP, Natarajan A, DeNardo SJ, Gervay-Hague J (2010) The remarkable stability of chimeric, sialic acid-derived alpha/delta-peptides in human blood plasma. Chem Biol Drug Des 75:455–460
Adams GP, DeNardo SJ, Deshpande SV, DeNardo GL, Meares CF, McCall MJ et al (1989) Effect of mass of 111In-benzyl-EDTA monoclonal antibody on hepatic uptake and processing in mice. Cancer Res 49:1707–1711
Schipper ML, Cheng Z, Lee SW, Bentolila LA, Iyer G, Rao J et al (2007) microPET-based biodistribution of quantum dots in living mice. J Nucl Med 48:1511–1518
Loening AM, Gambhir SS (2003) AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging 2:131–137
Kirschner AS, Ice RD, Beierwaltes WH (1973) Radiation dosimetry of 131I-19-iodocholesterol. J Nucl Med 14:713–717
Stabin MG, Siegel JA (2003) Physical models and dose factors for use in internal dose assessment. Heal Phys 85:294–310
Dunphy MP, Lewis JS (2009) Radiopharmaceuticals in preclinical and clinical development for monitoring of therapy with PET. J Nucl Med 50(Suppl 1):106S–121S
Dijkers EC, Kosterink JG, Rademaker AP, Perk LR, van Dongen GA, Bart J et al (2009) Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nucl Med 50:974–981
Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR et al (2010) Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 87:586–592
Conti PS, White C, Pieslor P, Molina A, Aussie J, Foster P (2005) The role of imaging with 111In-ibritumomab tiuxetan in the ibritumomab tiuxetan (zevalin) regimen: results from a zevalin imaging registry. J Nucl Med 46:1812–1818
Jalilian AR, Mirsadeghi L, Yari-Kamrani Y, Rowshanfarzad P, Kamali-Dehghan M, Sabet M (2007) Development of [Cu-64]-DOTA-anti-CD20 for targeted therapy. J Radioanal Nucl Chem 274:563–568
Chan C, Scollard D, McLarty K, Smith S, Reilly R (2011) A comparison of 111In- or 64Cu-DOTA-trastuzumab Fab fragments for imaging subcutaneous HER2-positive tumor xenografts in athymic mice using microSPECT/CT or microPET/CT. EJNMMI Res 1:15
Dixit R, Boelsterli UA (2007) Healthy animals and animal models of human disease(s) in safety assessment of human pharmaceuticals, including therapeutic antibodies. Drug Discov Today 12:336–342
Tsukamoto N, Kojima M, Hasegawa M, Oriuchi N, Matsushima T, Yokohama A et al (2007) The usefulness of (18)F-fluorodeoxyglucose positron emission tomography ((18)F-FDG-PET) and a comparison of (18)F-FDG-pet with (67)gallium scintigraphy in the evaluation of lymphoma: relation to histologic subtypes based on the World Health Organization classification. Cancer 110:652–659
(1996) Radiological protection and safety in medicine. Annals of the ICRP 26: 1–31
McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME et al (1998) Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 16:2825–2833
Smith-Jones PM, Solit D, Afroze F, Rosen N, Larson SM (2006) Early tumor response to Hsp90 therapy using HER2 PET: comparison with 18F-FDG PET. J Nucl Med 47:793–796
Olafsen T, Sirk SJ, Betting DJ, Kenanova VE, Bauer KB, Ladno W et al (2010) ImmunoPET imaging of B-cell lymphoma using 124I-anti-CD20 scFv dimers (diabodies). Protein Eng Des Sel 23:243–249
Karam M, Novak L, Cyriac J, Ali A, Nazeer T, Nugent F (2006) Role of fluorine-18 fluoro-deoxyglucose positron emission tomography scan in the evaluation and follow-up of patients with low-grade lymphomas. Cancer 107:175–183
Betting DJ, Kafi K, Abdollahi-Fard A, Hurvitz SA, Timmerman JM (2008) Sulfhydryl-based tumor antigen-carrier protein conjugates stimulate superior antitumor immunity against B cell lymphomas. J Immunol 181:4131–4140
Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L et al (2005) Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol 174:817–826
Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ (2007) Depletion of B cells in murine lupus: efficacy and resistance. J Immunol 179:3351–3361
Mease PJ (2008) B cell-targeted therapy in autoimmune disease: rationale, mechanisms, and clinical application. J Rheumatol 35:1245–1255
Acknowledgements
We thank Drs. Nicholas Van Bruggen at Genentech. We also thank Drs. Fred Chin, David Dick, and the staff in the radiochemistry and cyclotron facilities, the small animal imaging center, and Canary Center at Stanford for Cancer Early Detection for instrumentation support and analysis. This work was supported in part by grants from Genentech (South San Francisco, CA) and NCI ICMIC P50-CA114747 (SSG).
Conflict of interest disclosure
The authors declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Natarajan, A., Gowrishankar, G., Nielsen, C.H. et al. Positron Emission Tomography of 64Cu-DOTA-Rituximab in a Transgenic Mouse Model Expressing Human CD20 for Clinical Translation to Image NHL. Mol Imaging Biol 14, 608–616 (2012). https://doi.org/10.1007/s11307-011-0537-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-011-0537-8