Abstract
Purpose
Our previous studies with F-18-labeled anti-HER2 single-domain antibodies (sdAbs) utilized 5F7, which binds to the same epitope on HER2 as trastuzumab, complicating its use for positron emission tomography (PET) imaging of patients undergoing trastuzumab therapy. On the other hand, sdAb 2Rs15d binds to a different epitope on HER2 and thus might be a preferable vector for imaging in these patients. The aim of this study was to evaluate the tumor targeting of F-18 -labeled 2Rs15d in HER2-expressing breast carcinoma cells and xenografts.
Procedures
sdAb 2Rs15d was labeled with the residualizing labels N-succinimidyl 3-((4-(4-[18F]fluorobutyl)-1H-1,2,3-triazol-1-yl)methyl)-5-(guanidinomethyl)benzoate ([18F]RL-I) and N-succinimidyl 4-guanidinomethyl-3-[125I]iodobenzoate ([125I]SGMIB), and the purity and HER2-specific binding affinity and immunoreactivity were assessed after labeling. The biodistribution of I-125- and F-18-labeled 2Rs15d was determined in SCID mice bearing subcutaneous BT474M1 xenografts. MicroPET/x-ray computed tomograph (CT) imaging of [18F]RL-I-2Rs15d was performed in this model and compared to that of nonspecific sdAb [18F]RL-I-R3B23. MicroPET/CT imaging was also done in an intracranial HER2-positive breast cancer brain metastasis model after administration of 2Rs15d-, 5F7-, and R3B23-[18F]RL-I conjugates.
Results
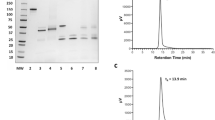
[18F]RL-I was conjugated to 2Rs15d in 40.8 ± 9.1 % yield and with a radiochemical purity of 97–100 %. Its immunoreactive fraction (IRF) and affinity for HER2-specific binding were 79.2 ± 5.4 % and 7.1 ± 0.4 nM, respectively. [125I]SGMIB was conjugated to 2Rs15d in 58.4 ± 8.2 % yield and with a radiochemical purity of 95–99 %; its IRF and affinity for HER2-specific binding were 79.0 ± 12.9 % and 4.5 ± 0.8 nM, respectively. Internalized radioactivity in BT474M1 cells in vitro for [18F]RL-I-2Rs15d was 43.7 ± 3.6, 36.5 ± 2.6, and 21.7 ± 1.2 % of initially bound radioactivity at 1, 2, and 4 h, respectively, and was similar to that seen for [125I]SGMIB-2Rs15d. Uptake of [18F]RL-I-2Rs15d in subcutaneous xenografts was 16–20 %ID/g over 1–3 h. Subcutaneous tumor could be clearly delineated by microPET/CT imaging with [18F]RL-I-2Rs15d but not with [18F]RL-I-R3B23. Intracranial breast cancer brain metastases could be visualized after intravenous administration of both [18F]RL-I-2Rs15d and [18F]RL-I-5F7.
Conclusions
Although radiolabeled 2Rs15d conjugates exhibited lower tumor cell retention both in vitro and in vivo than that observed previously for 5F7, given that it binds to a different epitope on HER2 from those targeted by the clinically utilized HER2-targeted therapeutic antibodies trastuzumab and pertuzumab, F-18-labeled 2Rs15d has potential for assessing HER2 status by PET imaging after trastuzumab and/or pertuzumab therapy.






Similar content being viewed by others
References
Schettini F, Buono G, Cardalesi C et al (2016) Hormone receptor/human epidermal growth factor receptor 2-positive breast cancer: where we are now and where we are going. Cancer Treat Rev 46:20–26
Santa-Maria CA, Nye L, Mutonga MB et al (2016) Management of metastatic HER2-positive breast cancer: where are we and where do we go from here? Oncology (Williston Park) 30:148–155
Recondo G Jr, de la Vega M, Galanternik F et al (2016) Novel approaches to target HER2-positive breast cancer: trastuzumab emtansine. Cancer Manag Res 8:57–65
Maximiano S, Magalhaes P, Guerreiro MP, Morgado M (2016) Trastuzumab in the treatment of breast cancer. BioDrugs 30:75–86
Gebhart G, Flamen P, De Vries EG et al (2016) Imaging diagnostic and therapeutic targets: human epidermal growth factor receptor 2. J Nucl Med 57(Suppl 1):81S–88S
Nitta H, Kelly BD, Allred C et al (2016) The assessment of HER2 status in breast cancer: the past, the present, and the future. Pathol Int 66:313–324
Karagoz Ozen DS, Ozturk MA, Aydin O et al (2014) Receptor expression discrepancy between primary and metastatic breast cancer lesions. Oncol Res Treat 37:622–626
Rack B, Zombirt E, Trapp E et al (2016) Comparison of HER2 expression in primary tumor and disseminated tumor cells in the bone marrow of breast cancer patients. Oncology 90:232–238
Kramer-Marek G, Oyen WJ (2016) Targeting the human epidermal growth factor receptors: imaging biomarkers from bench to bedside. J Nucl Med 57:996–1001
Sorensen J, Velikyan I, Sandberg D et al (2016) Measuring HER2-receptor expression in metastatic breast cancer using [68Ga]ABY-025 affibody PET/CT. Theranostics 6:262–271
Mendler CT, Gehring T, Wester HJ et al (2015) 89Zr-labeled versus 124I-labeled αHER2 Fab with optimized plasma half-life for high-contrast tumor imaging in vivo. J Nucl Med 56:1112–1118
Ma T, Sun X, Cui L et al (2014) Molecular imaging reveals trastuzumab-induced epidermal growth factor receptor downregulation in vivo. J Nucl Med 55:1002–1007
Olafsen T, Sirk SJ, Olma S et al (2012) ImmunoPET using engineered antibody fragments: fluorine-18 labeled diabodies for same-day imaging. Tumour Biol 33:669–677
Olafsen T, Kenanova VE, Sundaresan G et al (2005) Optimizing radiolabeled engineered anti-p185HER2 antibody fragments for in vivo imaging. Cancer Res 65:5907–5916
Kijanka M, Dorresteijn B, Oliveira S, van Bergen en Henegouwen PM (2015) Nanobody-based cancer therapy of solid tumors. Nanomedicine 10:161–174
De Meyer T, Muyldermans S, Depicker A (2014) Nanobody-based products as research and diagnostic tools. Trends Biotechnol 32:263–270
Keyaerts M, Xavier C, Heemskerk J et al (2016) Phase I study of 68Ga-HER2-nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J Nucl Med 57:27–33
Xavier C, Blykers A, Vaneycken I et al (2016) 18F-nanobody for PET imaging of HER2 overexpressing tumors. Nucl Med Biol 43:247–252
Vaidyanathan G, McDougald D, Choi J et al (2016) Preclinical evaluation of 18F-labeled anti-HER2 nanobody conjugates for imaging HER2 receptor expression by immuno-PET. J Nucl Med 57:967–973
Revets HM BC, Hoogenboom HM. (2011) Amino acid sequences directed against HER2 and polypeptides comprising the same for the treatment of cancers and/or tumors. US Patent 2011/0059090 Al
Rockberg J, Schwenk JM, Uhlen M (2009) Discovery of epitopes for targeting the human epidermal growth factor receptor 2 (HER2) with antibodies. Mol Oncol 3:238–247
Kramer-Marek G, Gijsen M, Kiesewetter DO et al (2012) Potential of PET to predict the response to trastuzumab treatment in an ErbB2-positive human xenograft tumor model. J Nucl Med 53:629–637
Vaneycken I, Devoogdt N, Van Gassen N et al (2011) Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. FASEB J 25:2433–2446
Xavier C, Vaneycken I, D’Huyvetter M et al (2013) Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 nanobodies for iPET imaging of HER2 receptor expression in cancer. J Nucl Med 54:776–784
Vaidyanathan G, McDougald D, Choi J et al (2016) N-Succinimidyl 3-((4-(4-[18F]fluorobutyl)-1H-1,2,3-triazol-1-yl)methyl)-5-(guanidinomethyl)ben zoate ([18F]SFBTMGMB): a residualizing label for 18F-labeling of internalizing biomolecules. Org Biomol Chem 14:1261–1271
Vaidyanathan G, Zalutsky MR (2007) Synthesis of N-succinimidyl 4-guanidinomethyl-3-[*I]iodobenzoate: a radio-iodination agent for labeling internalizing proteins and peptides. Nat Protoc 2:282–286
Pruszynski M, Koumarianou E, Vaidyanathan G et al (2013) Targeting breast carcinoma with radioiodinated anti-HER2 nanobody. Nucl Med Biol 40:52–59
Gray MA, Tao RN, DePorter SM et al (2016) A nanobody activation immunotherapeutic that selectively destroys HER2-positive breast cancer cells. Chembiochem 17:155–158
Lemaire M, D’Huyvetter M, Lahoutte T et al (2014) Imaging and radioimmunotherapy of multiple myeloma with anti-idiotypic nanobodies. Leukemia 28:444–447
Yu Z, Xia W, Wang HY et al (2006) Antitumor activity of an Ets protein, PEA3, in breast cancer cell lines MDA-MB-361DYT2 and BT474M1. Mol Carcinog 45:667–675
Choi J, Vaidyanathan G, Koumarianou E et al (2014) N-Succinimidyl guanidinomethyl iodobenzoate protein radiohalogenation agents: influence of isomeric substitution on radiolabeling and target cell residualization. Nucl Med Biol 41:802–812
Lindmo T, Boven E, Cuttitta F et al (1984) Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Meth 72:77–89
Kanojia D, Balyasnikova IV, Morshed RA et al (2015) Neural stem cells secreting anti-HER2 antibody improve survival in a preclinical model of HER2 overexpressing breast cancer brain metastases. Stem Cells 33:2985–2994
Velikyan I, Wennborg A, Feldwisch J et al (2016) Good manufacturing practice production of [68Ga]Ga-ABY-025 for HER2 specific breast cancer imaging. Am J Nucl Med Mol Imaging 6:135–153
Trousil S, Hoppmann S, Nguyen QD et al (2014) Positron emission tomography imaging with 18F-labeled ZHER2:2891 affibody for detection of HER2 expression and pharmacodynamic response to HER2-modulating therapies. Clin Cancer Res 20:1632–1643
Pruszynski M, Koumarianou E, Vaidyanathan G et al (2014) Improved tumor targeting of anti-HER2 nanobody through N-succinimidyl 4-guanidinomethyl-3-iodobenzoate radiolabeling. J Nucl Med 55:650–656
Sanchez-Crespo A (2013) Comparison of gallium-68 and fluorine-18 imaging characteristics in positron emission tomography. Appl Radiat Isot 76:55–62
D’Huyvetter M, Aerts A, Xavier C et al (2012) Development of 177Lu-nanobodies for radioimmunotherapy of HER2-positive breast cancer: evaluation of different bifunctional chelators. Contrast Media Mol Imaging 7:254–264
D’Huyvetter M, Vincke C, Xavier C et al (2014) Targeted radionuclide therapy with a 177Lu-labeled anti-HER2 nanobody. Theranostics 4:708–720
Banappagari S, McCall A, Fontenot K et al (2013) Design, synthesis and characterization of peptidomimetic conjugate of BODIPY targeting HER2 protein extracellular domain. Eur J Med Chem 65:60–69
Fuentes G, Scaltriti M, Baselga J, Verma CS (2011) Synergy between trastuzumab and pertuzumab for human epidermal growth factor 2 (Her2) from colocalization: an in silico based mechanism. Breast Cancer Res 13:R54
Xu FJ, Yu YH, Bae DS et al (1997) Radioiodinated antibody targeting of the HER-2/neu oncoprotein. Nucl Med Biol 24:451–459
Langmuir VK, Mendonca HL, Woo DV (1992) Comparisons between two monoclonal antibodies that bind to the same antigen but have differing affinities: uptake kinetics and 125I-antibody therapy efficacy in multicell spheroids. Cancer Res 52:4728–4734
Wargalla UC, Reisfeld RA (1989) Rate of internalization of an immunotoxin correlates with cytotoxic activity against human tumor cells. Proc Natl Acad Sci U S A 86:5146–5150
Ward DM, Kaplan J (1990) The rate of internalization of different receptor-ligand complexes in alveolar macrophages is receptor-specific. Biochem J 270:369–374
Stemmler HJ, Schmitt M, Harbeck N et al (2006) Application of intrathecal trastuzumab (Herceptin trademark) for treatment of meningeal carcinomatosis in HER2-overexpressing metastatic breast cancer. Oncol Rep 15:1373–1377
Stemmler HJ, Schmitt M, Willems A et al (2007) Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anti-Cancer Drugs 18:23–28
Dijkers EC, Oude Munnink TH, Kosterink JG et al (2010) Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 87:586–592
Acknowledgements
This work was supported in part by National Institutes of Health grants CA188177 and CA42324 and, for small-animal PET imaging, by S10RR31792. Excellent technical assistance of Elzbieta Krol (in vitro studies) and Xiao-guang Zhao (in vivo studies) is greatly appreciated. We also thank Thomas Hawk, Yulin Zhao, and Simone Degan for their excellent support with microPET/CT imaging studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhou, Z., Vaidyanathan, G., McDougald, D. et al. Fluorine-18 Labeling of the HER2-Targeting Single-Domain Antibody 2Rs15d Using a Residualizing Label and Preclinical Evaluation. Mol Imaging Biol 19, 867–877 (2017). https://doi.org/10.1007/s11307-017-1082-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-017-1082-x