Abstract
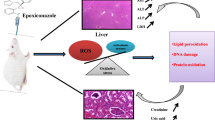
Water pollution has been a major concern for agrarian societies like Pakistan. Pharmaceutical industries are amongst the foremost contributor to industrial waste. Present study addresses the generation of oxidative stress caused by 2 months exposure to pharmaceutical wastewater in rats and their response to oral treatment with vitamin E, a potent antioxidant. The rats were randomized into five groups (n = 5) named as negative control, pharmaceutical wastewater (PEW) 100 %, PEW 10 %, PEW 1 %, and PEW 100 % + vitamin E. Oxidative damage in rats was evaluated by estimation of the activities of total superoxide dismutase (T-SOD), catalase (CAT), and the concentration of hydrogen peroxide (H2O2) in the liver, kidney, and blood/plasma. Exposure to pharmaceutical wastewater significantly decreased the activities of T-SOD and CAT and concentration of H2O2 in the liver and kidney and blood/plasma. Exposure to 100 % pharmaceutical wastewater exhibited a maximum decline in T-SOD activity, and activity was reduced to only 63.57 U/mL, 32.65, and 43.57 U/mg of protein in the plasma, kidney, and liver, respectively. Exposure to wastewater minimized activity CAT to 89.25 U/g of hemoglobin, 54.36, and 62.95 U/mg of protein in the blood, kidney, and liver, respectively. Treatment with vitamin E significantly increased the activity of T-SOD and CAT. However, increase in concentration of H2O2 was also observed in vitamin E exposed rats. Histopathology of the kidney revealed coagulative necrosis of renal epithelial cells and peritubular congestion. Endocardium showed infiltration of inflammatory cells and cellular breakdown in some areas. Lung sections exhibited atelectasis and emphysema of alveoli suggesting decline in lung function. The anatomy of the liver was also compromised due to severe degeneration and cellular swelling. The present study concluded that pharmaceutical wastewater induced severe oxidative stress in Wistar rats and ensued in histopathological lesions in several vital organs suggesting its high toxicity. Non-enzymatic antioxidant vitamin E may ameliorate oxidative stress induced by pharmaceutical wastewater.

Similar content being viewed by others
References
Akhtar MF, Ashraf M, Anjum AA, Javeed A, Sharif A, Saleem A, Akhtar B (2016a) Textile industrial effluent induces mutagenicity and oxidative DNA damage and exploits oxidative stress biomarkers in rats. Environ Toxicol Pharmacol 41:180–186. doi:10.1016/j.etap.2015.11.022
Akhtar MF et al (2016b) Toxicity appraisal of untreated dyeing industry wastewater based on chemical characterization and short term bioassays. Bull Environ Contam Toxicol 96:502–507. doi:10.1007/s00128-016-1759-x
Andreozzi R, Marotta R, Pinto G, Pollio A (2002) Carbamazepine in water: persistence in the environment, ozonation treatment and preliminary assessment on algal toxicity. Water Res 36:2869–2877
Andreozzi R et al (2004) Effects of advanced oxidation processes (AOPs) on the toxicity of a mixture of pharmaceuticals. Water Science & Technology 50:23–28
Banos G, Medina-Campos ON, Maldonado PD, Zamora J, Pérez I, Pavón N, Pedraza-Chaverrí J (2005) Antioxidant enzymes in hypertensive and hypertriglyceridemic rats: effect of gender. Clin Exp Hypertens 27:45–57
Barbier O, Jacquillet G, Tauc M, Cougnon M, Poujeol P (2005) Effect of heavy metals on, and handling by, the kidney. Nephron Physiology 99:105–p110
Chakraborti A, Gulati K, Banerjee BD, Ray A (2007) Possible involvement of free radicals in the differential neurobehavioral responses to stress in male and female rats. Behav Brain Res 179:321–325
Chaudiere J, Ferrari-Iliou R (1999) Intracellular antioxidants: from chemical to biochemical mechanisms. Food Chem Toxicol 37:949–962
Cui X et al (2004) Subchronic exposure to arsenic through drinking water alters expression of cancer-related genes in rat liver. Toxicol Pathol 32:64–72
Ejaz S, Chekarova I, Cho JW, Lee SY, Ashraf S, Lim CW (2009) Effect of aged garlic extract on wound healing: a new frontier in wound management. Drug Chem Toxicol 32:191–203
El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH (2004) Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and β-carotene. Food Chem Toxicol 42:1563–1571
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1:529–539. doi:10.2174/1568026013394831
Gadipelly C, Pérez-González A, Yadav GD, Ortiz I, Ibáñez R, Rathod VK, Marathe KV (2014) Pharmaceutical industry wastewater: review of the technologies for water treatment and reuse. Ind Eng Chem Res 53:11571–11592
Jadhav S, Sarkar S, Tripathi H (2006) Cytogenetic effects of a mixture of selected metals following subchronic exposure through drinking water in male rats. Indian J Exp Biol 44:997
Jadhav S, Sarkar S, Patil R, Tripathi H (2007a) Effects of subchronic exposure via drinking water to a mixture of eight water-contaminating metals: a biochemical and histopathological study in male rats. Arch Environ Contam Toxicol 53:667–677
Jadhav SH, Sarkar SN, Kataria M, Tripathi HC (2007b) Subchronic exposure to a mixture of groundwater-contaminating metals through drinking water induces oxidative stress in male rats. Environ Toxicol Pharmacol 23:205–211
Jemec A, Drobne D, Tišler T, Sepčić K (2010) Biochemical biomarkers in environmental studies—lessons learnt from enzymes catalase, glutathione S-transferase and cholinesterase in two crustacean species. Environ Sci Pollut Res 17:571–581
Ji J, Wu Z, Liu Q, Zhang Y, Ye M, Li M (1991) An ultramicroanalytic and rapid method for determination of superoxide dismutase activity. Journal of Nanjing Railway Medical College 10:27–29
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283:65–87
Jurczuk M, Brzóska MM, Moniuszko-Jakoniuk J, Gałażyn-Sidorczuk M, Kulikowska-Karpińska E (2004) Antioxidant enzymes activity and lipid peroxidation in liver and kidney of rats exposed to cadmium and ethanol. Food Chem Toxicol 42:429–438
Kim I, Tanaka H (2010) Use of ozone-based processes for the removal of pharmaceuticals detected in a wastewater treatment plant. Water Environment Research 82:294–301
Kudłak B, Namieśnik J (2008) Environmental fate of endocrine disrupting compounds—analytical problems and challenges. Crit Rev Anal Chem 38:242–258
Lakshmi B, Sudhakar M, Aparna M (2013) Protective potential of black grapes against lead induced oxidative stress in rats. Environ Toxicol Pharmacol 35:361–368
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Luo Z, Wang B, Liu M, Jiang K, Liu M, Wang L (2014) Effect of dietary supplementation of vitamin C on growth, reactive oxygen species, and antioxidant enzyme activity of Apostichopus japonicus (Selenka) juveniles exposed to nitrite. Chin J Oceanol Limnol 32:749–763
Madden EF, Fowler BA (2000) Mechanisms of nephrotoxicity from metal combinations: a review. Drug Chem Toxicol 23:1–12
Mates J (2000) Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 153:83–104
Michałowicz J, Duda W (2007) Phenols—sources and toxicity. Pol J Environ Stud 16:347–362
Mousa SA (2004) Expression of adhesion molecules during cadmium hepatotoxicity. Life Sci 75:93–105
Olas B, Wachowicz B (2002) Resveratrol and vitamin C as antioxidants in blood platelets. Thromb Res 106:143–148
Onyema OO, Farombi EO, Emerole GO, Ukoha AI, Onyeze GO (2006) Effect of vitamin E on monosodium glutamate induced hepatotoxicity and oxidative stress in rats. Indian J Biochem Biophys 43:20
Patlolla AK, Barnes C, Yedjou C, Velma V, Tchounwou PB (2009) Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague-Dawley rats. Environ Toxicol 24:66–73
Ramm GA (2005) Ruddell RG Hepatotoxicity of iron overload: mechanisms of iron-induced hepatic fibrogenesis. In: Seminars in liver disease. vol 4. pp 433–449
Rao MV, Parekh SS, Chawla SL (2006) Vitamin-E supplementation ameliorates chromium-and/or nickel induced oxidative stress in vivo. J Health Sci 52:142–147
Santra A, Das Gupta J, De B, Roy B, Guha Mazumder D (1999) Hepatic manifestations in chronic arsenic toxicity. Indian J Gastroenterol 18:152–155
Sengupta T, Chattopadhyay D, Ghosh N, Das M, Chatterjee G (1990) Effect of chromium administration on glutathione cycle of rat intestinal epithelial cells. Indian J Exp Biol 28:1132–1135
Shaikh ZA, Vu TT, Zaman K (1999) Oxidative stress as a mechanism of chronic cadmium-induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol Appl Pharmacol 154:256–263
Sharif A et al (2016) Pharmaceutical wastewater being composite mixture of environmental pollutants may be associated with mutagenicity and genotoxicity. Environ Sci Pollut Res 23:2813–2820
Singh MP, Reddy MK, Mathur N, Saxena D, Chowdhuri DK (2009) Induction of hsp70, hsp60, hsp83 and hsp26 and oxidative stress markers in benzene, toluene and xylene exposed Drosophila melanogaster: role of ROS generation. Toxicol Appl Pharmacol 235:226–243
Sirtori C, Zapata A, Oller I, Gernjak W, Agüera A, Malato S (2009) Decontamination industrial pharmaceutical wastewater by combining solar photo-Fenton and biological treatment. Water Res 43:661–668
Smythies J (1999) The neurotoxicity of glutamate, dopamine, iron and reactive oxygen species: functional interrelationships in health and disease: a review—discussion. Neurotox Res 1:27–39
Susa N, Ueno S, Furukawa Y, Sugiyama M (1996) Protective effect of vitamin E on chromium (VI)-induced cytotoxicity and lipid peroxidation in primary cultures of rat hepatocytes. Arch Toxicol 71:20–24
Valko M, Rhodes C, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160:1–40
Xu Z et al (2013) Protective effects of selenium on oxidative damage and oxidative stress related gene expression in rat liver under chronic poisoning of arsenic. Food Chem Toxicol 58:1–7
Zaidi SKR, Banu N (2004) Antioxidant potential of vitamins A, E and C in modulating oxidative stress in rat brain. Clin Chim Acta 340:229–233
Zhang Y, Luo Y, Hou Y-X, Jiang H, Chen Q, Tang H-R (2008) Chilling acclimation induced changes in the distribution of H2O2 and antioxidant system of strawberry leaves. Agric J 3:286–291
Acknowledgments
The authors wish to thank Chairman Department of Pharmacology and Toxicology for his kind support and valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the experimental protocols were performed in compliance with Institutional Guidelines for the Care and Use of Laboratory Animals, UVAS, Lahore, Pakistan.
Funding
The research received no specific grant from any funding agency in public, commercial, or not-for-profit sectors.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Sharif, A., Ashraf, M., Javeed, A. et al. Oxidative stress responses in Wistar rats on subacute exposure to pharmaceutical wastewater. Environ Sci Pollut Res 23, 24158–24165 (2016). https://doi.org/10.1007/s11356-016-7717-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7717-7