Abstract
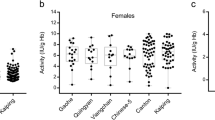
The prevalence of glucose-6-phosphate dehydrogenase (G6PD) deficiency and its gene mutations were studied in the Achang population from Lianghe County in Southwestern China. We found that 7.31% (19 of 260) males and 4.35% (10 of 230) females had G6PD deficiency. The molecular analysis of G6PD gene exons 2–13 was performed by a PCR-DHPLC-Sequencing or PCR-Sequencing. Sixteen independent subjects with G6PD Mahidol (487G>A) and the new polymorphism IVS5-612 (G>C), which combined into a novel haplotype, were identified accounting for 84.2% (16/19). And 100% Achang G6PD Mahidol were linked to the IVS5-612 C. The percentage of G6PD Mahidol in the Achang group is close to that in the Myanmar population (91.3% 73/80), which implies that there are some gene flows between Achang and Myanmar populations. Interestingly, G6PD Canton (1376G>T) and G6PD Kaiping (1388G>A), which were the most common G6PD variants from other ethnic groups in China, were not found in this Achang group, suggesting that there are different G6PD mutation profiles in the Achang group and other ethnic groups in China. Our findings appear to be the first documented report on the G6PD genetics of the AChang people, which will provide important clues to the Achang ethnic group origin and will help prevention and treatment of malaria in this area.
Similar content being viewed by others
References
Pandolfi P P, Sonati F, Rivi R, et al. Targeted disruption of the house-keeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J, 1995, 14(21): 5209–5215
Sabeti P C, Reich D E, Higgins J M, et al. Detecting recent positive selection in the human genome from haplotype structure. Nature, 2002, 419(6909): 832–837
Volkman S, Barry A, Lyons E, et al. Recent origin of plasmodium falciparum from a single progenitor. Science, 2001, 293(5529): 482–484
WHO and UNICEF. A 5-minutes briefing on the World. Malaria Report, 2005
Li Z X, Zhou J J, Shen Z R, et al. Identification and expression profiling of putative odorant-binding proteins in the malaria mosquitoes, Anopheles gambiae and A. arabiensis. Sci China Ser C-Life Sci, 2004, 47(6): 567–576
Yang J. The prevalence and distribution of malaria in Dehongzhou Prefecture in 1951–2001. China Trop Med (in Chinese), 2004, 4(5): 756–758
Li H, Yang Y, Jiang H. Malaria epidemic and outbreak situation in Yunnan Province in 2003. Parasitizes and Infectious Diseases (in Chinese), 2005, 3(4): 152–156
Beutler E. G6PD deficiency. Blood, 1994, 84(11): 3613–3636
Vulliamy T J, Kaeda J S, Ait-Chafa D. et al. Clinical and hematological consequences of recurrent G6PD mutations and a single new mutation causing chronic nonspherocytic haemolytic anaemia. Br J Haematol, 1998, 101(4): 670–675
Kotaka M, Gover S, Rutten L V, et al. Structural studies of glucose-6-phosphate and NADP(+) binding to human glucose-6-phosphate dehydrogenase. Acta Crystallogr D Biol Crystallogr, 2005, 61(Pr 5): 495–504
Jiang, W Y, Yu G L, Liu P. et al. Structure and function of glucose 6-phosphate dehydrogenase-deficient variants in Chinese population. Hum Genet, 2006, 119(5): 463–478
Du C S, Xu Y K, Hu X Y. Favism (in Chinese). Bejing: People’s Medical Publishing House, 1987, 189–193
Betke K, Beutler E, Brewer G J, et al. Standardization of procedures for the study of glucose-6-phosphate dehydrogenase. WHO Tech Rep Ser, 1967, 366: 1–53
Bruggen R V, Bautista J M, Petropoulou T, et al. Deletion of leucine 61 in glucose-6-phosphate dehydrogenase leads to chronic non-spherocytic anemia, granulocyte dysfunction, and increased susceptibility to infection. Blood, 2002, 100(3): 1026–1030
Jiang W, Chen L, Lin Q, et al. Denaturing high-performance liquid chromatography technique platform applied to screen G6PD deficient variants. Chin J Med Genet, 2005, 22(6): 607–611
Tseng C P, Huang D L, Chong K Y, et al. Rapid detection of glucose-6-phosphate dehydrogenase gene mutations by denaturing high-performancae liquid chromatography. Clinical Biochem, 2005, 38(11): 973–980
Nuchprayoon I, Sanpavat S, Nuchprayoon S. Glucose-6-phosphate Dehydrogenase (G6PD) mutations in Thailand: G6PD Viangchan (871G>A) is the most common deficiency variant in the Thai population. Human Mutation, 2002, 19(2): 185–190
Matsuoka H, Wang J, Hirai M, et al. Glucose-6-phosphate dehydrogenase (G6PD) mutations in Myanmar: G6PD Mahidol (487G>A) is the most common variant in the Myanmar population. J Hum Genet, 2004, 49(10): 544–547
Panish V, Sungnate T, Wasi P, et al. G-6-PD Mahidol: The most common glucose-6-phosphate dehydrogenase variant in Thailand. J Med Assoc Thailand, 1972, 55: 576–585
Vulliamy T J, Wanachiwanawint W, Mason P J, et al. G6PD Mahidol, a common deficient variant in South East Asia is caused by a (163)glycine-serine mutation. Nucleic Acids Research, 1989(22), 17: 5868
Iwai K, Hirono A, Matsuoka H, et al. Distribution of glucose-6-phosphate dehydrogenase mutations in Southeast Asia. Hum Genet, 2001, 108(6): 445–449
Laosombat V, Sattayasevana B. Molecular heterogeneity of glucose-6-phosphate dehydrogenase (G6PD) variants in the south of Thailand and identification of a novel variant (G6PD Songklanagarind). Blood Cells Mol Dies, 2005, 34(2): 191–196
Anion O, Yu Y H, Amir-Muhriz A L, et al. Glucose-6-phosphate dehydrogenase (G6PD) variants in Malaysian Malays. Human Mutation, 2003, 21(1): 101–109
Jing W, Du C, Chen L, et al. Study on G487A mutation of the glucose-6-phosphate dehydrogenase gene. Chin J Hematol (in Chinese), 1999, 20: 518–520
Yang Z, Chu J, Ban G, et al. The genotype analysis of glucose-6-phosphate dehydrogenase deficiency in Yunnan Province. Chin J Med Genet (in Chinese), 2001, 18: 259–263
Tishkoff S A, Varkonyi T, Cahinhinan N, et al. Haplotype diversity and linkage disequilibrium at human G6PD: recent origin of alleles that confer malarial resistance. Science, 2001, 293(5529): 455–462
Matsuoka H, Nguon C, Kanbe T, et al. Glucose-6-phosphate dehydrogenase (G6PD) mutations in Cambodia: G6PD Viangchan (871G→A) is the most common variant in the Cambodian population. J Hum Genet, 2005, 50(9): 468–478
Rahimi Z, Vaisi-Raygani A, Nagel R L, et al. Molecular characterization of glucose-6-phosphate dehydrogenase deficiency in the Kurdish population of Western Iran. Blood Cells Mol Dis, 2006, 37(2): 91–94
Kang L C, Zhao X R, Zhou Y S, et al. Mutations analysis of STK11gene in Chinese families with Peutz-Jeghers syndrome. Chin Sci Bull, 2003, 48(4): 333–337
Liu M R, Lu Y Y. Frequent mtDNA mutations and its role in gastric carcinogenesis. Chin Sci Bull, 2002, 47(20): 1720–1724
Ffranze A, Ferrante M I, Fusco F, et al. Molecular anatomy of the human glucose-6-phosphate dehydrogenase core promoter. FEBS Lett, 1998, 437(3): 313–318
Menounos P G, Garini G A, Patrinos G P. Glucose-6-phosphate dehydrogenase deficiency does not result from mutations in the promoter region of the G6PD gene. J Clinical Lab Anal, 2003, 17(3): 90–92
Phtlippe M, Larondelle Y, Lemaigre F, et al. Promoter function of the human glucose-6-phosphate dehydrogenase gene depends on two GC boxes that are cell specifically controlled. Eur J Biochem, 1994, 226(2): 377–384
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work
Supported by the National Natural Science Foundation of China (Grant No. 30460049)
Rights and permissions
About this article
Cite this article
Yang, Y., Zhu, Y., Li, D. et al. Characterization of glucose-6-phosphate dehydrogenase deficiency and identification of a novel haplotype 487G>A/IVS5-612(G>C) in the Achang population of Southwestern China. SCI CHINA SER C 50, 479–485 (2007). https://doi.org/10.1007/s11427-007-0072-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11427-007-0072-7