Summary
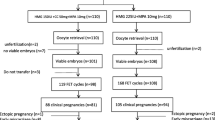
Polycystic ovary syndrome (PCOS) is one of the most common causes of infertility in women. Progestin-primed ovarian stimulation (PPOS) protocol, which used oral progestin to prevent premature luteinizing hormone (LH) surges in ovarian stimulation, has been proved to be effective and safe in patients with PCOS. The aim of the present study was to compare the efficacy of PPOS protocol with that of the traditional gonadotropin-releasing hormone (GnRH) antagonist protocol in patients with PCOS. A total of 157 patients undergoing in-vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) were recruited into this study. The patients were divided into two groups by the stimulation protocols: the GnRH antagonist protocol group and the PPOS protocol group. There was no significant difference in the clinical characteristics between the two groups. Dose and duration of gonadotropin were higher in the PPOS protocol group. Estradiol levels on the day of human chorionic gonadotropin (hCG) administration were significantly lower in the PPOS protocol group. Fertilization rates and the number of good quality embryos were similar between the two groups. Remarkably, we found 6 patients with moderate ovarian hyperstimulation syndrome (OHSS) in the GnRH antagonist protocol group but 0 in the PPOS protocol group. A total of 127 women completed their frozen embryo transfer (FET) cycles. There were no significant differences between the two groups in terms of clinical pregnancy rate per transfer, implantation rate, first-trimester miscarriage rate and on-going pregnancy rate per transfer. To conclude, PPOS protocol decreased the incidence of OHSS without adversely affecting clinical outcomes in patients with PCOS.
Similar content being viewed by others
References
Thessaloniki, Eshre Asrm-Sponsored Pcos Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod, 2008,23:462–477
Boyle JA, Cunningham J, O’Dea K, et al. Prevalence of polycystic ovary syndrome in a sample of Indigenous women in Darwin, Australia. Med J Aust, 2012,196(1):62–66
Ma Y, Li R, Qiao J, et al. Characteristics of abnormal menstrual cycle and polycystic ovary syndrome in community and hospital populations. Chin Med J, 2010,123(16):2185–2189
Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod, 2004,19(1):41–47
Balen AH, Morley LC, Misso M, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update, 2016,22(6):687–708
Kuang Y, Chen Q, Fu Y, et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril, 2015,104(1):62–70.e3
Crha I, Ventruba P, Filipinská E, et al. Medroxy-progesteron acetate use to block LH surge in oocyte donor stimulation. Ceska Gynekol, 2018,83(1):11–16
Kuang Y, Hong Q, Chen Q, et al. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil Steril, 2014,101(1):105–111
Wang Y, Chen Q, Wang N, et al. Controlled Ovarian Stimulation Using Medroxyprogesterone Acetate and hMG in Patients With Polycystic Ovary Syndrome Treated for IVF: A Double-Blind Randomized Crossover Clinical Trial. Medicine (Baltimore), 2016,95(9):e2939
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod, 2011,26(6):1270–1283
Schmidt DW, Maier DB, Nulsen JC, et al. Reducing the dose of human chorionic gonadotropin in high responders does not affect the outcomes of in vitro fertilization. Fertil Steril, 2004,82(4):841–846
Xiao Z, Zhou X, Xu W, et al. Natural cycle is superior to hormone replacement therapy cycle for vitrificated-preserved frozen-thawed embryo transfer. Syst Biol Reprod Med, 2012,58(2):107–112
Mourad S, Brown J, Farquhar C. Interventions for the prevention of OHSS in ART cycles: an overview of Cochrane reviews. Cochrane Database Syst Rev, 2017,23,1:CD012103
Toftager M, Sylvest R, Schmidt L, et al. Quality of life and psychosocial and physical well-being among 1,023 women during their first assisted reproductive technology treatment: secondary outcome to a randomized controlled trial comparing gonadotropin-releasing hormone (GnRH) antagonist and GnRH agonist protocols. Fertil Steril, 2018,109(1):154–164
Yu S, Long H, Chang HY, et al. New application of dydrogesterone as a part of a progestin-primed ovarian stimulation protocol for IVF: a randomized controlled trial including 516 first IVF/ICSI cycles. Hum Reprod, 2018,33(2):229–237
Wang N, Lin J, Zhu Q, et al. Comparison of neonatal outcomes and live-birth defects after progestin-primed ovarian stimulation versus conventional ovarian stimulation for in vitro fertilization: A large retrospective cohort study. Medicine (Baltimore), 2018,97(34):e11906
Zhu X, Ye H, Fu Y. Use of Utrogestan during controlled ovarian hyperstimulation in normally ovulating women undergoing in vitro fertilization or intracytoplasmic sperm injection treatments in combination with a “freeze all” strategy: a randomized controlled dose-finding study of 100 mg versus 200 mg. Fertil Steril, 2017,107(2):379–386.e4
Chen Q, Wang Y, Sun L, et al. Controlled ovulation of the dominant follicle using progestin in minimal stimulation in poor responders. Reprod Biol Endocrinol, 2017,15(1):71
Yucel O, Ekin M, Cengiz H, et al. Comparison of estradiol and progesterone priming/antagonist/letrozole and microdose flare-up protocols for poor responders undergoing intracytoplasmic sperm injection. Gynecol Endocrinol, 2014,30(9):653–656
Roque M, Valle M, Guimarães F, et al. Freeze-all policy: fresh vs. frozen-thawed embryo transfer. Fertil Steril, 2015,103(5):1190–1193
Meng Y, Guo Y, Qian Y, et al. Effects of GnRH antagonist on endometrial protein profiles in the window of implantation. Proteomics, 2014,14(20):2350–2359
Griesinger G, Venetis CA, Marx T, et al. Oral contraceptive pill pretreatment in ovarian stimulation with GnRH antagonists for IVF: a systematic review and meta-analysis. Fertil Steril, 2008,90(4):1055–1063
Fatemi HM, Doody K, Griesinger G, et al. High ovarian response does not jeopardize ongoing pregnancy rates and increases cumulative pregnancy rates in a GnRH-antagonist protocol. Hum Reprod, 2013,28(2):442–452
Bosch E, Labarta E, Pellicer A. Does cumulative live birth plateau beyond a certain ovarian response? Fertil Steril, 2017,108(6):943
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Natural Science Foundation of China (Nos. 81471455, 81100418).
Rights and permissions
About this article
Cite this article
Xiao, Zn., Peng, Jl., Yang, J. et al. Flexible GnRH Antagonist Protocol versus Progestin-primed Ovarian Stimulation (PPOS) Protocol in Patients with Polycystic Ovary Syndrome: Comparison of Clinical Outcomes and Ovarian Response. CURR MED SCI 39, 431–436 (2019). https://doi.org/10.1007/s11596-019-2055-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-019-2055-x