Abstract
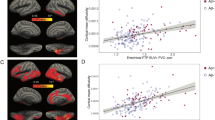
Neurofibrillary tangles (NFT) and amyloid plaques are hallmark neuropathological features of Alzheimer’s disease (AD). There is some debate as to which neuropathological feature comes first in the disease process, with early autopsy studies suggesting that NFT develop first, and more recent neuroimaging studies supporting the early role of amyloid beta (Aβ) deposition. Cerebrospinal fluid (CSF) biomarkers of Aβ42 and hyperphosphorylated tau (p-tau) have been shown to serve as in vivo proxy measures of amyloid plaques and NFT, respectively. The aim of this study was to examine the association between CSF biomarkers and rate of atrophy in the precuneus and hippocampus. These regions were selected because the precuneus appears to be affected early and severely by Aβ deposition, and the hippocampus similarly by NFT pathology. We predicted (1) baseline Aβ42 would be related to accelerated rate of cortical thinning in the precuneus and volume loss in the hippocampus, with the latter relationship expected to be weaker, (2) baseline p-tau181p would be related to accelerated rate of hippocampal atrophy and cortical thinning in the precuneus, with the latter relationship expected to be weaker. Using all ADNI cohorts, we fitted separate linear mixed-effects models for changes in hippocampus and precuneus longitudinal outcome measures with baseline CSF biomarkers modeled as predictors. Results partially supported our hypotheses: Both baseline p-tau181p and Aβ42 were associated with hippocampal atrophy over time. Neither p-tau181p nor Aβ42 were significantly related to cortical thinning in the precuneus over time. However, follow-up analyses demonstrated that having abnormal levels of both Aβ42 and p-tau181p was associated with an accelerated rate of atrophy in both the hippocampus and precuneus. Results support early effects of Aβ in the Alzheimer’s disease process, which are less apparent than and perhaps dependent on p-tau effects as the disease progresses. However, amyloid deposition alone may be insufficient for emergence of significant morphometric changes and clinical symptoms.

Similar content being viewed by others
References
Aizenstein, H. J., Nebes, R. D., Saxton, J. A., Price, J. C., Mathis, C. A., Tsopelas, N. D., et al. (2008). Frequent amyloid deposition without significant cognitive impairment among the elderly. Archives of Neurology, 65(11), 1509–1517.
Apostolova, L. G., Hwang, K. S., Andrawis, J. P., Green, A. E., Babakchanian, S., Morra, J. H., et al. (2010). 3D PIB and CSF biomarker associations with hippocampal atrophy in ADNI subjects. Neurobiology of Aging, 31(8), 1284–1303.
Becker, J. A., Hedden, T., Carmasin, J., Maye, J., Rentz, D. M., Putcha, D., et al. (2011). Amyloid-beta associated cortical thinning in clinically normal elderly. Annals of Neurology, 69(6), 1032–1042.
Beckett, L. A., Harvey, D. J., Gamst, A., Donohue, M., Kornak, J., Zhang, H., et al. (2010). The Alzheimer’s disease neuroimaging initiative: annual change in biomarkers and clinical outcomes. Alzheimer’s & Dementia, 6(3), 257–264.
Blennow, K., & Hampel, H. (2003). CSF markers for incipient Alzheimer’s disease. Lancet Neurology, 2(10), 605–613.
Braak, H., Braak, E., Bohl, J., & Reintjes, R. (1996). Age, neurofibrillary changes, a beta-amyloid and the onset of Alzheimer’s disease. Neuroscience Letters, 210(2), 87–90.
Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38.
Buckner, R. L., Head, D., Parker, J., Fotenos, A. F., Marcus, D., Morris, J. C., et al. (2004). A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage, 23(2), 724–738.
Buckner, R. L., Snyder, A. Z., Shannon, B. J., LaRossa, G., Sachs, R., Fotenos, A. F., et al. (2005). Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. Journal of Neuroscience, 25(34), 7709–7717.
Buerger, K., Ewers, M., Pirttila, T., Zinkowski, R., Alafuzoff, I., Teipel, S. J., et al. (2006). CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain, 129(Pt 11), 3035–3041.
Caroli, A., & Frisoni, G. B. (2010). The dynamics of Alzheimer’s disease biomarkers in the Alzheimer’s disease neuroimaging initiative cohort. Neurobiology of Aging, 31(8), 1263–1274.
Chetelat, G., Villemagne, V. L., Bourgeat, P., Pike, K. E., Jones, G., Ames, D., et al. (2010). Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Annals of Neurology, 67(3), 317–324.
Clark, C. M., Xie, S., Chittams, J., Ewbank, D., Peskind, E., Galasko, D., et al. (2003). Cerebrospinal fluid tau and beta-amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Archives of Neurology, 60(12), 1696–1702.
Conover, W. J., & Inman, R. L. (1981). Rank transformations as a bridge between parametric and nonparametric statistics. The American Statistician, 35, 124–129.
Dale, A. M., Fischl, B., & Sereno, M. I. (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179–194.
de Leon, M. J., DeSanti, S., Zinkowski, R., Mehta, P. D., Pratico, D., Segal, S., et al. (2006). Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiology of Aging, 27(3), 394–401.
Desikan, R. S., McEvoy, L. K., Thompson, W. K., Holland, D., Roddey, J. C., Blennow, K., et al. (2011). Amyloid-beta associated volume loss occurs only in the presence of phospho-tau. Annals of Neurology, 70(4), 657–661.
Desikan, R. S., Segonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980.
Diggle, P. J., Heagerty, P., Liang, K.-Y., & Zeger, S. L. (2002). Analysis of longitudinal data (2nd ed.). Oxford: Oxford University Press.
Dorfel, D., Werner, A., Schaefer, M., von Kummer, R., & Karl, A. (2009). Distinct brain networks in recognition memory share a defined region in the precuneus. European Journal of Neuroscience, 30(10), 1947–1959.
Fagan, A. M., Mintun, M. A., Shah, A. R., Aldea, P., Roe, C. M., Mach, R. H., et al. (2009). Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer’s disease. EMBO Molecular Medicine, 1(8–9), 371–380.
Fennema-Notestine, C., Hagler, D. J., Jr., McEvoy, L. K., Fleisher, A. S., Wu, E. H., Karow, D. S., et al. (2009). Structural MRI biomarkers for preclinical and mild Alzheimer’s disease. Human Brain Mapping, 30(10), 3238–3253.
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355.
Fischl, B., Salat, D. H., van der Kouwe, A. J., Makris, N., Segonne, F., Quinn, B. T., et al. (2004). Sequence-independent segmentation of magnetic resonance images. NeuroImage, 23(Suppl 1), S69–S84.
Fischl, B., Sereno, M. I., & Dale, A. M. (1999). Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage, 9(2), 195–207.
Fischl, B., van der Kouwe, A., Destrieux, C., Halgren, E., Segonne, F., Salat, D. H., et al. (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14(1), 11–22.
Fjell, A. M., Walhovd, K. B., Amlien, I., Bjornerud, A., Reinvang, I., Gjerstad, L., et al. (2008). Morphometric changes in the episodic memory network and tau pathologic features correlate with memory performance in patients with mild cognitive impairment. AJNR. American Journal of Neuroradiology, 29(6), 1183–1189.
Fjell, A. M., Walhovd, K. B., Fennema-Notestine, C., McEvoy, L. K., Hagler, D. J., Holland, D., et al. (2010). CSF biomarkers in prediction of cerebral and clinical change in mild cognitive impairment and Alzheimer’s disease. Journal of Neuroscience, 30(6), 2088–2101.
Folstein, M. F., Robins, L. N., & Helzer, J. E. (1983). The mini-mental state examination. Archives of General Psychiatry, 40(7), 812.
Hampel, H., Burger, K., Pruessner, J. C., Zinkowski, R., DeBernardis, J., Kerkman, D., et al. (2005). Correlation of cerebrospinal fluid levels of tau protein phosphorylated at threonine 231 with rates of hippocampal atrophy in Alzheimer disease. Archives of Neurology, 62(5), 770–773.
Hampel, H., Goernitz, A., & Buerger, K. (2003). Advances in the development of biomarkers for Alzheimer’s disease: from CSF total tau and Abeta(1–42) proteins to phosphorylated tau protein. Brain Research Bulletin, 61(3), 243–253.
Hardy, J. (2009). The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. Journal of Neurochemistry, 110(4), 1129–1134.
Hardy, J. A., & Higgins, G. A. (1992). Alzheimer’s disease: the amyloid cascade hypothesis. Science, 256(5054), 184–185.
Henneman, W. J., Vrenken, H., Barnes, J., Sluimer, I. C., Verwey, N. A., Blankenstein, M. A., et al. (2009). Baseline CSF p-tau levels independently predict progression of hippocampal atrophy in Alzheimer disease. Neurology, 73(12), 935–940.
Hyman, B. T. (2011). Amyloid-dependent and amyloid-independent stages of Alzheimer disease. Archives of Neurology, 68(8), 1062–1064.
Jack, C. R., Jr., Knopman, D. S., Jagust, W. J., Shaw, L. M., Aisen, P. S., Weiner, M. W., et al. (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurology, 9(1), 119–128.
Jagust, W. J., Bandy, D., Chen, K., Foster, N. L., Landau, S. M., Mathis, C. A., et al. (2010). The Alzheimer’s disease neuroimaging initiative positron emission tomography core. Alzheimer’s & Dementia, 6(3), 221–229.
Josephs, K. A., Whitwell, J. L., Ahmed, Z., Shiung, M. M., Weigand, S. D., Knopman, D. S., et al. (2008). Beta-amyloid burden is not associated with rates of brain atrophy. Annals of Neurology, 63(2), 204–212.
Kobayashi, Y., & Amaral, D. G. (2003). Macaque monkey retrosplenial cortex: II. Cortical afferents. The Journal of Comparative Neurology, 466(1), 48–79.
Lo, R. Y., Hubbard, A. E., Shaw, L. M., Trojanowski, J. Q., Petersen, R. C., Aisen, P. S., et al. (2011). Longitudinal change of biomarkers in cognitive decline. Archives of Neurology, 68(10), 1257–1266.
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., & Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology, 34(7), 939–944.
Mintun, M. A., Larossa, G. N., Sheline, Y. I., Dence, C. S., Lee, S. Y., Mach, R. H., et al. (2006). [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology, 67(3), 446–452.
Mormino, E. C., Kluth, J. T., Madison, C. M., Rabinovici, G. D., Baker, S. L., Miller, B. L., et al. (2009). Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain, 132(Pt 5), 1310–1323.
Morris, J. C. (1993). The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology, 43(11), 2412–2414.
Nelson, P. T., Abner, E. L., Scheff, S. W., Schmitt, F. A., Kryscio, R. J., Jicha, G. A., et al. (2009). Alzheimer’s-type neuropathology in the precuneus is not increased relative to other areas of neocortex across a range of cognitive impairment. Neuroscience Letters, 450(3), 336–339.
Petersen, R. C., Aisen, P. S., Beckett, L. A., Donohue, M. C., Gamst, A. C., Harvey, D. J., et al. (2010). Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology, 74(3), 201–209.
Petersen, R. C., Doody, R., Kurz, A., Mohs, R. C., Morris, J. C., Rabins, P. V., et al. (2001). Current concepts in mild cognitive impairment. Archives of Neurology, 58(12), 1985–1992.
Rabinovici, G. D., & Jagust, W. J. (2009). Amyloid imaging in aging and dementia: testing the amyloid hypothesis in vivo. Behavioural Neurology, 21(1), 117–128.
Rowe, C. C., Ng, S., Ackermann, U., Gong, S. J., Pike, K., Savage, G., et al. (2007). Imaging beta-amyloid burden in aging and dementia. Neurology, 68(20), 1718–1725.
Schuff, N., Woerner, N., Boreta, L., Kornfield, T., Shaw, L. M., Trojanowski, J. Q., et al. (2009). MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain, 132(Pt 4), 1067–1077.
Shaw, L. M., Korecka, M., Clark, C. M., Lee, V. M., & Trojanowski, J. Q. (2007). Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nature Reviews. Drug Discovery, 6(4), 295–303.
Shaw, L. M., Vanderstichele, H., Knapik-Czajka, M., Clark, C. M., Aisen, P. S., Petersen, R. C., et al. (2009). Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Annals of Neurology, 65(4), 403–413.
Sheline, Y. I., Morris, J. C., Snyder, A. Z., Price, J. L., Yan, Z., D’Angelo, G., et al. (2010). APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. Journal of Neuroscience, 30(50), 17035–17040.
Sheline, Y. I., Raichle, M. E., Snyder, A. Z., Morris, J. C., Head, D., Wang, S., et al. (2010). Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biological Psychiatry, 67(6), 584–587.
Sperling, R. A., Laviolette, P. S., O’Keefe, K., O’Brien, J., Rentz, D. M., Pihlajamaki, M., et al. (2009). Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron, 63(2), 178–188.
Strozyk, D., Blennow, K., White, L. R., & Launer, L. J. (2003). CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology, 60(4), 652–656.
Teipel, S. J., Bokde, A. L., Meindl, T., Amaro, E., Jr., Soldner, J., Reiser, M. F., et al. (2010). White matter microstructure underlying default mode network connectivity in the human brain. NeuroImage, 49(3), 2021–2032.
Tosun, D., Schuff, N., Truran-Sacrey, D., Shaw, L. M., Trojanowski, J. Q., Aisen, P., et al. (2010). Relations between brain tissue loss, CSF biomarkers, and the ApoE genetic profile: a longitudinal MRI study. Neurobiology of Aging, 31(8), 1340–1354.
Trojanowski, J. Q., Vandeerstichele, H., Korecka, M., Clark, C. M., Aisen, P. S., Petersen, R. C., et al. (2010). Update on the biomarker core of the Alzheimer’s disease neuroimaging initiative subjects. Alzheimer’s & Dementia, 6(3), 230–238.
Vemuri, P., Wiste, H. J., Weigand, S. D., Knopman, D. S., Trojanowski, J. Q., Shaw, L. M., et al. (2010). Serial MRI and CSF biomarkers in normal aging, MCI, and AD. Neurology, 75(2), 143–151.
Acknowledgments
This manuscript was a collaborative effort from the 2011 Friday Harbor Advanced Psychometrics Workshop, funded by the National Institute on Aging R13 AG030995. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott; Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Amorfix Life Sciences Ltd.; AstraZeneca; Bayer HealthCare; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals Inc.; Eli Lilly and Company; F. Hoffmann-LaRoche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129, K01 AG030514, P30 AG008017, R01 AG029672-01A1 and the Dana Foundation.
Disclosures
There were no actual or potential conflicts of interest for any of the authors.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
For the Alzheimer’s Disease Neuroimaging Initiative—Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.ucla.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.ucla.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Rights and permissions
About this article
Cite this article
Stricker, N.H., Dodge, H.H., Dowling, N.M. et al. CSF biomarker associations with change in hippocampal volume and precuneus thickness: implications for the Alzheimer’s pathological cascade. Brain Imaging and Behavior 6, 599–609 (2012). https://doi.org/10.1007/s11682-012-9171-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-012-9171-6