Abstract
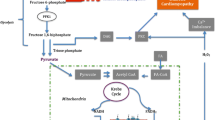
Diabetes results in a cardiomyopathy characterized by reduced contractility that is primarily the result of changes in calcium handling within the myocyte. Because most of the calcium involved in excitation-contraction (EC) coupling is derived from the sarcoplasmic reticulum (SR), it is no surprise that many studies have found a reduction in sarcoendoplasmic reticulum calcium ATPase (SERCA) activity in the diabetic state. In this review, we outline the changes to SR calcium handling in the diabetic state and, through the use of transgenic mice and adenoviral gene therapy, we examine how SR function can be improved by the expression of various proteins that are directly and indirectly involved in calcium handling. Improving SERCA activity plays an important role in ameliorating the contractile phenotype associated with the diabetic state.
Similar content being viewed by others
References and Recommended Reading
Regan TJ, Lyons MM, Ahmed SS, et al.: Evidence for cardiomyopathy in familial diabetes mellitus. J Clin Invest 1977, 60:884–899.
Fein FS: Diabetic cardiomyopathy. Diabetes Care 1990, 13:1169–1179.
Penpargkul S, Fein FS, Sonnenblick EH, et al.: Depressed cardiac sarcoplasmic reticulum function from diabetic rats. J. Mol Cell Cardiol 1981, 13:303–309.
Bouchard RA, Bose D: Influence of experimental diabetes on sarcoplasmic reticulum function in rat ventricular muscle. Am J Physiol 260:H341–H354.
Lagadic-Gossmann D, Buckler KJ, Le Prigent K, et al.: Altered Ca2+ handling in ventricular myocytes isolated from diabetic rats. Am J Physiol 1996, 270:H1529-H1537.
Hansford RG: Physiological role of mitochondrial Ca2+ transport. J Bioengerg Biomembr 1994, 26:495–508.
Davidoff AJ, Mason MM, Davidson MB, et al.: Sucrose-induced cardiomyocyte dysfunction is both preventable and reversible with clinically relevant treatments. Am J Physiol Endocrinol Metab 2004, 286:E718-E724.
Belke DD, Betuing S, Tuttle MJ, et al.: Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest 2002, 109:629–639. This is the first study to examine cardiac function in mice engineered to lack insulin signaling specifically within the myocyte itself without the confounding affect of diabetes systemically.
Aasum E, Hafstad AD, Severson DL, et al.: Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes 2003, 52:434–442.
Yu JZ, Rodrigues B, McNeill JH: Intracellular calcium levels are unchanged in the diabetic heart. Cardiovasc Res 1997, 34:91–98.
Dhalla NS, Liu X, Panagia V, et al.: Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res 1998, 40:239–247.
Choi KM, Zhong Y, Hoit BD, et al.: Defective intracellular Ca2+ signaling contributes to cardiomyopathy in type 1 diabetes. Am J Physiol Heart Circ Physiol 2002, 283:H1398-H1408.
Arikawa M, Takahashi N, Hara M, et al.: Enhanced inhibition of L-type calcium currents by troglitazone in streptozotocininduced diabetic rat cardiac ventricular myocytes. Br J Pharmacol 2002, 136:803–810.
Trost SU, Belke DD, Bluhm WF, et al.: Overexpression of the sarcoplasmic reticulum Ca2+-ATPase improves myocardial contractility in diabetic cardiomyopathy. Diabetes 2002, 52:1166–1171.
Kim HW, Ch YS, Lee HR, et al.: Diabetic alterations in cardiac sarcoplasmic reticulum Ca2+-ATPase and phospholamban protein expression. Life Sci 2001, 70:367–379.
Zhong Y, Ahmed S, Grupp IL, et al.: Altered SR protein expression associated with contractile dysfunction in diabetic rat hearts. Am J Physiol Heart Circ Physiol 2001, 281:H1137-H1147.
Netticadan T, Temsah RM, Kent A, et al.: Depressed levels of Ca2+-cycling proteins may underlie sarcoplasmic reticulum dysfunction in the diabetic heart. Diabetes 2001, 50:2133–2138.
Russ M, Reinauer H, Eckel J: Diabetes-induced decrease in the mRNA coding for sarcoplasmic reticulum Ca2+-ATPase in adult rat cardiomyocytes. Biochem Biophys Res Commun 1991, 178:906–912.
Han X, Abendschein DR, Kelly JG, et al.: Diabetes-induced changes in specific lipid molecular species in rat myocardium. Biochem J 2000, 352:79–89.
Xu KY, Zweier JL, Becker LC: Functional coupling between glycolysis and sarcoplasmic reticulum Ca2+ transport. Circ Res 1995, 77:88–97
Vetter R, Rehfeld U, Reissfelder C, et al.: Transgenic overexpression of the sarcoplasmic reticulum Ca2+-ATPase improves reticulate Ca2+ handling in normal and diabetic rat hearts. FASEB J 2002, 16:1657–1659. This reference (and Trost et al. [14]) provides direct evidence of the importance of SERCA expression in playing a role in mediating the contractile phenotype in the diabetic state.
Brittsan AG, Carr AN, Schmidt AG, et al.: Maximal inhibition of SERCA2 Ca2+ affinity by phospholamban in transgenic hearts overexpressing a non-phosphorylatable form of phospholamban. J Biol Chem 2000, 275:12129–12135.
Kadambi VJ, Ponniah S, Herrer JM, et al.: Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice. J Clin Invest 1996, 97:533–539.
Santana LF, Kranias EG, Lederer WJ: Calcium sparks and excitation-contraction coupling in phospholamban-deficient mouse ventricular myocytes. J Physiol 1997, 503:21–29.
Brittsan AG, Kiss E, Edes I, et al.: The effect of isoproterenol on phospholamban-deficient mouse hearts with altered thyroid conditions. J Mol Cell Cardiol 1999, 31:1725–1737.
Haghighi K, Kolokathis F, Pater L, et al.: Human phospholamban null results in lethal dialated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest 2003, 111:801–803.
Schmitt JP, Kamisago M, Asahi M, et al.: Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science 2003, 299:1410–1413.
Haghighi K, Schmidt AG, Hoit BD, et al.: Superinhibition of sarcoplasmic reticulum function by phospholamban induces cardiac contractile failure. J Biol Chem 2001, 276:24145–24152.
Atkins FL, Dowell RT, Love S: Beta-adrenergic receptors, adenylate cyclase activity, and cardiac dysfunction in the rat. J Cardiovasc Pharamacol 1985, 7:66–70.
Packer M: The development of positive inotropic agents for chronic heart failure: How have we gone astray? J Am Coll Cardiol 1993, 22:119A-126A.
Lefkowitz RJ, Rockman HA, Koch WJ: Catecholamines, cardiac beta-adrenergic receptors, and heart failure. Circulation 2000, 101:1634–1637.
Suzuki T, Wang JH: Stimulation of bovine cardiac sarcoplasmic reticulum Ca2+ pump and blocking of phospholamban phosphorylation and dephosphorylation by a phospholamban monoclonal antibody. J Biol Chem 1986, 261:7018–7023.
Sham JS, Jones LR, Morad M: Phospholamban mediates the beta-adrenergic-enhanced Ca2+ uptake in mammalian ventricular myocytes. Am J Physiol 1991, 261:H1344-H1349.
Meyer M, Belke DD, Trost SU, et al.: A recombinant antibody increases cardiac contractility by mimicking phospholamban phosphorylation. FASEB J 2004, 18:1312–1314. This study provides evidence that an antibody expressed within cardiac myocytes can favorably alter SERCA activity to improve cardiac function in a diabetic state. The idea of expressing antibodies intracellularly to alter protein function represents a new therapeutic avenue in treating heart disease.
Most P, Remppis A, Pleger ST, et al.: Transgenic overexpression of the Ca2+-binding protein S100A1 in the heart leads to increased in vivo myocardial contractile performance. J Biol Chem 2003, 278:33809–33817.
Maki M, Narayana SV, Hitomi K: A growing family of the Ca2+-binding proteins with five EF-hand motifs. Biochem J 1997, 328:718–720.
Meyers MB, Pickel VM, Sheu SS, et al.: Association of sorcin with the cardiac ryanodine receptor. J Biol Chem 1995, 270:26411–26418.
Farrell EF, Antaramian A, Rueda A, et al.: Sorcin inhibits calcium release and modulates excitation-contraction coupling in the heart. J Biol Chem 2003, 278:34660–34666.
Lokuta AJ, Meyers MB, Sander PR, et al.: Modulation of cardiac ryanodine receptors by sorcin. J Biol Chem 1997, 272:25333–25338.
Seidler T, Miller SL, Loughrey CM, et al.: Effects of adenovirusmediated sorcin overexpression on excitation-contraction coupling in isolated rabbit cardiomyocytes. Circ Res 2003, 93:132–139.
Meyers MB, Fischer A, Sun YJ, et al.: Sorcin regulates excitationcontraction coupling in the heart. J Biol Chem 2003, 278:28865–28871.
Shannon TR, Pogwizd SM, Bers DM: Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res 2003, 93:592–594.
Suarez J, Belke DD, Gloss B, et al.: In vivo adenoviral transfer of sorcin reverses cardiac contractile abnormalities of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 2004, 286:H68-H75.
Bidasee KR, Zhang Y, Shao CH, et al.: Diabetes increases formation of advanced glycation end products on sarco(endo)plasmic reticulum Ca2+-ATPase. Diabetes 2004, 53:463–473.
Bidasee KR, Nallani K, Yu Y, et al.: Chronic diabetes increases advanced glycation end products on cardiac ryanodine receptors/calcium-release channels. Diabetes 2003, 52:1825–1836.
Ren J, Gintant GA, Miller RE, et al.: High extracellular glucose impairs cardiac E-C coupling in a glycosylation-dependent manner. Am J Physiol 1997, 273:H2876-H2883.
Parker GJ, Lund KC, Taylor RP, et al.: Insulin resistance of glycogen synthase mediated by O-linked N-acetylglucosamine. J Biol Chem 2003, 278:10022–10027.
Marsall S, Garvey WT, Traxinger RR: New insights into the metabolic regulation of insulin action and insulin resistance: role of glucose and amino acids. FASEB J 1991, 5:3031–3036.
Vosseller K, Wells L, Lane MD, et al.: Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci USA 2002, 99:5313–5318.
Clark RJ, McDonough PM, Swanson E, et al.: Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem 2003, 278:44230–44237. This study provides evidence for a direct link between exposure to high glucose and a downregulation in SERCA expression through Olinked glycosylation of transcription factors.
Baker DL, Dave V, Reed T, et al.: Multiple Sp1 binding sites in the cardiac/slow twitch muscle sarcoplasmic reticulum Ca2+-ATPase gene promoter are required for expression in SoL8 muscle cells. J Biol Chem 1996, 271:5921–5928.
Yang X, Su K, Roos MD, et al.: O-linkage of N-acetylglucosamine to Sp1 activation domain inhibits its transcriptional capability. Proc Natl Acad Sci USA 2001, 98:6611–6616.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Belke, D.D., Dillmann, W.H. Altered cardiac calcium handling in diabetes. Current Science Inc 6, 424–429 (2004). https://doi.org/10.1007/s11906-004-0035-3
Issue Date:
DOI: https://doi.org/10.1007/s11906-004-0035-3