Abstract
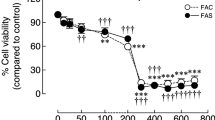
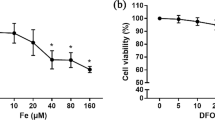
Bone metabolism has a close relationship with iron homeostasis. To examine the effects of iron excess and iron deficiency on the biological activities of osteoblast in vitro, human osteoblast cells (hFOB1.19) were incubated in a medium supplemented with 0–200 μmol/L ferric ammonium citrate and 0–20 μmol/L deferoxamine. The intracellular iron was measured by a confocal laser scanning microscope. Proliferation of osteoblasts was evaluated by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide assay. Apoptotic cells were detected using annexin intervention V/PI staining with a flow cytometry. Alkaline phosphatase (ALP) activity was measured using an ALP assay kit. The number of calcified nodules and mineral area was evaluated by von Kossa staining assay. The expressions of type I collagen and osteocalcin of cultured osteoblasts were detected by reverse transcriptase polymerase chain reaction and Western blot. Intracellular reactive oxygen species (ROS) was measured using the oxidation-sensitive dye 2,7-dichlorofluorescin diacetate by flow cytometry. The results indicated that excessive iron inhibited osteoblast activity in a concentration-dependent manner. Low iron concentrations, in contrast, produced a biphasic manner on osteoblasts: mild low iron promoted osteoblast activity, but serious low iron inhibited osteoblast activity. Osteogenesis was optimal in certain iron concentrations. The mechanism underlying biological activity invoked by excessive iron may be attributed to increased intracellular ROS levels.








Similar content being viewed by others
Abbreviations
- FAC:
-
Ferric ammonium citrate
- DFO:
-
Deferoxamine
- ALP:
-
Alkaline phosphatase
- COL-I:
-
Type I collagen
- OC:
-
Osteocalcin
- RT-PCR:
-
Reverse transcriptase polymerase chain reaction
- ROS:
-
Reactive oxygen species
References
Anderson GJ, Frazer DM, McLaren GD (2009) Iron absorption and metabolism. Curr Opin Gastroenterol 25(2):129–135. doi:10.1097/MOG.0b013e32831ef1f7
Stangl GI, Kirchgessner M (1998) Different degrees of moderate iron deficiency modulate lipid metabolism of rats. Lipids 33(9):889–895. doi:10.1007/s11745-998-0285-8
Galaris D, Mantzaris M, Amorgianiotis C (2008) Oxidative stress and aging: the potential role of iron. Hormones (Athens) 7(2):114–122
Tuomainen T-P, Loft S, Nyyssonen K, Punnonen K, Salonen JT, Poulsen HE (2007) Body iron is a contributor to oxidative damage of DNA. Free Radic Res 41(3):324–328. doi:10.1080/10715760601091642
Weinberg ED (2009) Iron availability and infection. Biochim Biophys Acta 1790(7):600–605. doi:10.1016/j.bbagen.2008.07.002
Weinberg ED (2006) Iron loading: a risk factor for osteoporosis. Biometals 19(6):633–635. doi:10.1007/s10534-006-9000-8, An international journal on the role of metal ions in biology, biochemistry, and medicine
Weinberg ED (2008) Role of iron in osteoporosis. Pediatr Endocrinol Rev 6(Suppl 1):81–85
Guggenbuhl P, Deugnier Y, Boisdet JF, Rolland Y, Perdriger A, Pawlotsky Y, Chales G (2005) Bone mineral density in men with genetic hemochromatosis and HFE gene mutation. Osteoporos Int 16(12):1809–1814. doi:10.1007/s00198-005-1934-0, A journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA
Morabito N, Russo GT, Gaudio A, Lasco A, Catalano A, Morini E, Franchina F, Maisano D, La Rosa M, Plota M, Crifo A, Meo A, Frisina N (2007) The “lively” cytokines network in beta-thalassemia major-related osteoporosis. Bone 40(6):1588–1594. doi:10.1016/j.bone.2007.02.020
Sarrai M, Duroseau H, D’Augustine J, Moktan S, Bellevue R (2007) Bone mass density in adults with sickle cell disease. Br J Haematol 136(4):666–672. doi:10.1111/j.1365-2141.2006.06487.x
Skordis N, Toumba M (2011) Bone disease in thalassaemia major: recent advances in pathogenesis and clinical aspects. Pediatr Endocrinol Rev 8(Suppl 2):300–306
Valenti L, Varenna M, Fracanzani AL, Rossi V, Fargion S, Sinigaglia L (2009) Association between iron overload and osteoporosis in patients with hereditary hemochromatosis. Osteoporos Int 20(4):549–555. doi:10.1007/s00198-008-0701-4
Isomura H, Fujie K, Shibata K, Inoue N, Iizuka T, Takebe G, Takahashi K, Nishihira J, Izumi H, Sakamoto W (2004) Bone metabolism and oxidative stress in postmenopausal rats with iron overload. Toxicology 197(2):93–100. doi:10.1016/j.tox.2003.12.006
Kudo H, Suzuki S, Watanabe A, Kikuchi H, Sassa S, Sakamoto S (2008) Effects of colloidal iron overload on renal and hepatic siderosis and the femur in male rats. Toxicology 246(2–3):143–147. doi:10.1016/j.tox.2008.01.004
Tsay J, Yang Z, Ross FP, Cunningham-Rundles S, Lin H, Coleman R, Mayer-Kuckuk P, Doty SB, Grady RW, Giardina PJ, Boskey AL, Vogiatzi MG (2010) Bone loss caused by iron overload in a murine model: importance of oxidative stress. Blood 116(14):2582–2589. doi:10.1182/blood-2009-12-260083
Abraham R, Walton J, Russell L, Wolman R, Wardley-Smith B, Green JR, Mitchell A, Reeve J (2006) Dietary determinants of post-menopausal bone loss at the lumbar spine: a possible beneficial effect of iron. Osteoporos Int 17(8):1165–1173. doi:10.1007/s00198-005-0033-6
Maurer J, Harris MM, Stanford VA, Lohman TG, Cussler E, Going SB, Houtkooper LB (2005) Dietary iron positively influences bone mineral density in postmenopausal women on hormone replacement therapy. J Nutr 135(4):863–869
Katsumata S, Tsuboi R, Uehara M, Suzuki K (2006) Dietary iron deficiency decreases serum osteocalcin concentration and bone mineral density in rats. Biosci Biotechnol Biochem 70(10):2547–2550
Katsumata SI, Katsumata-Tsuboi R, Uehara M, Suzuki K (2009) Severe iron deficiency decreases both bone formation and bone resorption in rats. J Nutr 139(2):238–243. doi:10.3945/jn.108.093757
Doyard M, Fatih N, Monnier A, Island ML, Aubry M, Leroyer P, Bouvet R, Chales G, Mosser J, Loreal O, Guggenbuhl P (2012) Iron excess limits HHIPL-2 gene expression and decreases osteoblastic activity in human MG-63 cells. Osteoporos Int. doi:10.1007/s00198-011-1871-z, A journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA
Messer JG, Kilbarger AK, Erikson KM, Kipp DE (2009) Iron overload alters iron-regulatory genes and proteins, down-regulates osteoblastic phenotype, and is associated with apoptosis in fetal rat calvaria cultures. Bone 45(5):972–979. doi:10.1016/j.bone.2009.07.073
Yamasaki K, Hagiwara H (2009) Excess iron inhibits osteoblast metabolism. Toxicol Lett 191(2–3):211–215. doi:10.1016/j.toxlet.2009.08.023
Messer JG, Cooney PT, Kipp DE (2010) Iron chelator deferoxamine alters iron-regulatory genes and proteins and suppresses osteoblast phenotype in fetal rat calvaria cells. Bone 46(5):1408–1415. doi:10.1016/j.bone.2010.01.376
Garcia JJ, Martinez-Ballarin E, Millan-Plano S, Allue JL, Albendea C, Fuentes L, Escanero JF (2005) Effects of trace elements on membrane fluidity. J Trace Elem Med Biol 19(1):19–22. doi:10.1016/j.jtemb.2005.07.007
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283(2–3):65–87. doi:10.1016/j.tox.2011.03.001
Fatokun AA, Stone TW, Smith RA (2008) Responses of differentiated MC3T3-E1 osteoblast-like cells to reactive oxygen species. Eur J Pharmacol 587(1–3):35–41. doi:10.1016/j.ejphar.2008.03.024
Mody N, Parhami F, Sarafian TA, Demer LL (2001) Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med 31(4):509–519
Kicic A, Chua AC, Baker E (2001) Effect of iron chelators on proliferation and iron uptake in hepatoma cells. Cancer 92(12):3093–3110
Zarjou A, Jeney V, Arosio P, Poli M, Zavaczki E, Balla G, Balla J (2010) Ferritin ferroxidase activity: a potent inhibitor of osteogenesis. J Bone Miner Res: Off J Am Soc Bone Miner Res 25(1):164–172. doi:10.1359/jbmr.091002
Cornish J, Callon KE, Naot D, Palmano KP, Banovic T, Bava U, Watson M, Lin JM, Tong PC, Chen Q, Chan VA, Reid HE, Fazzalari N, Baker HM, Baker EN, Haggarty NW, Grey AB, Reid IR (2004) Lactoferrin is a potent regulator of bone cell activity and increases bone. Formation in vivo. Endocrinology 145(9):4366–4374. doi:10.1210/en.2003-1307
Liu G, Men P, Kenner GH, Miller SC (2006) Age-associated iron accumulation in bone: implications for postmenopausal osteoporosis and a new target for prevention and treatment by chelation. Biometals: Int J Role Metal Ions Biol, Biochem, Med 19(3):245–251. doi:10.1007/s10534-005-6666-2
Liu G, Men P, Kenner GH, Miller SC (2008) Therapeutic effects of an oral chelator targeting skeletal tissue damage in experimental postmenopausal osteoporosis in rats. Hemoglobin 32(1–2):181–190. doi:10.1080/03630260701726707
Pattanapanyasat K, Webster HK, Tongtawe P, Kongcharoen P, Hider RC (1992) Effect of orally active hydroxypyridinone iron chelators on human lymphocyte function. Br J Haematol 82(1):13–19. doi:10.1111/j.1365-2141.1992.tb04587.x
Reichard P, Ehrenberg A (1983) Ribonucleotide reductase—a radical enzyme. Science 221(4610):514–519
Qu ZH, Zhang XL, Tang TT, Dai KR (2008) Promotion of osteogenesis through beta-catenin signaling by desferrioxamine. Biochem Bioph Res Co 370(2):332–337. doi:10.1016/j.bbrc.2008.03.092
Suarez IG, Martin JLF, Diaz MN, Andia JBC (2003) Effect of desferrioxamine and deteriprone on the 1,25(OH)(2)D-3-stimulated osteocalcin secretion in osteoblast-like cells. Nefrologia 23:27–31
Ishii KA, Fumoto T, Iwai K, Takeshita S, Ito M, Shimohata N, Aburatani H, Taketani S, Lelliott CJ, Vidal-Puig A, Ikeda K (2009) Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med 15(3):259–266. doi:10.1038/nm.1910
Acknowledgments
This work was partially supported by the National Natural Science Foundation of China (no. 81273090), Jiangsu provincial grant (no. BK2012608), and science and technology projects of Suzhou (no. 510303).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, Gy., Zhao, Lp., He, Yf. et al. A Comparison of the Biological Activities of Human Osteoblast hFOB1.19 Between Iron Excess and Iron Deficiency. Biol Trace Elem Res 150, 487–495 (2012). https://doi.org/10.1007/s12011-012-9511-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9511-9