Abstract
Although surfactin is able to inhibit cancer cell proliferation and to induce cancer cell apoptosis, the molecular mechanism responsible for this process remain elusive. In this study, the signaling network underlying the apoptosis of human hepatoma (HepG2) cells induced by surfactin was investigated. It is found that the reaction oxygen species (ROS) production and intracellular calcium ([Ca2+]i) accumulation are both induced HepG2 cells apoptosis. The [Ca2+]i exaltation was partly depended on the Ca2+ release from inositol 1,4,5-trisphosphate (IP3) and ryanodine (Ry) receptors channels, which both triggered endoplasmic reticulum stress (ERS). The results showed that surfactin induced the ROS production and ROS production led to ERS. The occurrence of ERS increased the [Ca2+]i level and the processes associated with blocking extracellular signal-regulated kinase (ERK) pathway. According to a comprehensive review of all the evidence, it is concluded that surfactin induces apoptosis of HepG2 cells through a ROS–ERS–Ca2+ mediated ERK pathway.
Similar content being viewed by others
Introduction
Surfactin is a bacterial cyclic lipopeptide produced by Bacillus subtilis [14]. In the course of various studies of its properties, surfactin was found to exhibit effective characteristics like anti-bacterial, anti-viral, anti-tumor, anti-mycoplasma, and hemolytic activities [15].
Intracellular calcium ([Ca2+]i) is a critical second messenger and regulator of cell apoptosis, a change in cytosolic free Ca2+ leads to cell apoptosis through several downstream mechanisms [16, 20]. [Ca2+]i increases probably due to direct activation of the receptor and generation of plasmalemmal Ca2+ influx. Disruption of Ca2+ homeostasis, in turn, induces a series of biochemical reactions that result in caspase activation and subsequent cell apoptosis [2, 8].
Ca2+ concentration can inhibit folded protein capacity of the endoplasmic reticulum (ER) and result in the accumulation of unfolded protein a condition termed as the endoplasmic reticulum stress (ERS) [1]. When ERS is prolonged, C/EBP-homologous transcription factor (CHOP/Gadd153) is upregulated downstream of the basic leucine zipper (bZIP) transcription factor activating transcription factor 4 (ATF4) and uniquely responsive to ERS [11]. CHOP/Gadd153 causes down regulation of the anti-apoptotic protein B cell lymphoma-2 (Bcl-2), cytochrome c release and caspase-3 activation [3, 5, 6]. To date, it is not well documented how the surfactin triggers ER Ca2+ release channels, then causes the collapse of Ca2+ homeostasis in surfactin-induced apoptosis.
Reaction oxygen species (ROS) may play an important role as a second messenger and may lead to the activation of mitogen-activated protein kinases (MAPKs) [12, 17, 18]. Another important issue was that both [Ca2+]i increase and ROS were involved in cell apoptosis, it would be interesting to know which of these phenomena occurred firstly, whether or not they have an effect on MAPKs.
Our previous study have indicated that elevation [Ca2+]i and mitochondrial are associated with the development of apoptosis [4]. However, the mechanism of generating the Ca2+ response with surfactin is not clearly clarified. In this study, the effect of surfactin on disruption of Ca2+ homeostasis and Ca2+-dependent enzymes in HepG2 were studied. We investigated the possibility whether intracellular ROS, ERS, Ca2+, and MAPKs pathway are involved in surfactin-induced apoptosis of HepG2 cells.
Materials and Methods
Materials
Surfactin from Bacillus natto TK-1 was purified as previously described [4]. Mouse polyclonal antibodies against β-actin, CHOP/Gadd153, immunoglobulin heavy chain binding protein/glucose-regulated protein 78 (Bip/GRP78), p-ERK and horseradish peroxidase conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell Culture
HepG2 cell lines were obtained from the Institute of Biochemistry and cell Biology (Shanghai, China). The cells were maintained in minimum essential medium alpha medium (DMEM, high glucose) supplemented with 10 % heat inactivated fetal bovine serum at 37 °C in a humidified chamber of 95 % air and 5 % CO2.
Measurement of Intracellular ROS Production
The intracellular generation of ROS was inspected by 2′,7′-dichlorofluorescein diacetate (DCFH-DA). DCFH-DA is deacetylated by intracellular esterase and converted to nonfluorescent dichlorodihydrofluorescein, which is oxidized rapidly to the highly fluorescent compound dichlorofluorescein in the presence of ROS. HepG2 cells were incubated with 42 μg/ml surfactin for 2, 4, and 6 h and loaded with 10 μM DCFH-DA for 30 min at 37 °C in the dark. Then, cells were washed with phosphate buffer saline (PBS) (pH 7.2) three times. The fluorescence was measured by laser scatter confocal microscope (LSCM) using an excitation of 488 nm and an emission of 525 nm.
Measurement of Calcium Concentration
HepG2 cells were incubated with 42 μg/ml surfactin for 3, 9, and 12 h. The levels of [Ca2+]i was measured by Fluo-3/AM, a visible wavelength calcium probe. The dye was added to the HepG2 cells for 1 h at 37 °C under 5 % CO2 in the dark. Then, the cells were washed three times with PBS (pH 7.2). The fluorescence was measured by LSCM using an excitation of 488 nm and an emission of 525 nm.
SDS-PAGE and Western Blotting Analysis
SDS-PAGE and western blotting analysis have been described previously [19]. In brief, HepG2 cells were lysed and quantified, 10 μg of protein per lane was loaded on 10 % SDS-PAGE and transferred to nitrogen cellular membrane. The membrane was blocked and incubated with primary antibody. After washed with PBS, the membrane was incubated with the secondary antibody. Densitometry of the western blots was done using QuantiScan software (Cambridge, UK).
Statistical Analysis
Experiments were performed at least three times with similar results and results were expressed as the mean ± SEM. Statistical comparisons were performed with SPSS 17.0 (Statistical Product and Service Solutions USA).
Results
Measurement of Intracellular ROS Production
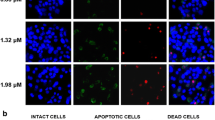
To investigate the effect of ROS production on HepG2 cells apoptosis, intracellular ROS production was detected. As shown in Fig. 1, there was visual change of fluorescence at 2 h compared to control, the fluorescence reached the peak value at 4 h and decreased at 6 h compared to 4 h. To demonstrate whether ROS participates in surfactin-induced apoptosis, the inhibition rate of HepG2 cells treated with surfactin in the presence or absence of N-acetyl-l-cysteine (NAC, inhibitor of ROS) was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). The results showed the inhibition rate of cells pretreated with NAC was evidently decreased compared with cells treated with surfactin alone (data was not shown), these results confirmed that ROS generation was involved in the surfactin-induced apoptosis in HepG2 cells (Fig. 1).
Effect of ROS on ERS
Bip/GRP78 and CHOP/Gadd153 were considered to be important unfolded protein in ER, and both of them were ER residential chaperone and ER stress hallmark. As shown in Fig. 2, the expression of both Bip/GRP78 and CHOP/Gadd153 increased in a time-dependent manner after being treated with surfactin. The expression of Bip/GRP78 and CHOP/Gadd153 both decreased pretreated with NAC compared to being treated with surfactin alone (p < 0.01). This indicated that the activation of ERS was regulated by ROS production (Fig. 2).
Measurement of [Ca2+]i Production
To detect if surfactin has an effect on the cytoplasmic Ca2+ concentration, [Ca2+]i was measured. As shown in Fig. 3A, when treated with surfactin, [Ca2+]i was significantly increased, and the increase of [Ca2+]i was in a time-dependent manner. To determine whether the [Ca2+]i accumulated in cytoplasm finally induced cell apoptosis, 1,2-bis-(o-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid, tetraacetoxymethyl esteris (BAPTA-AM, a chelator of calcium) was used to pretreated cells prior to adding surfactin, and cell inhibition rate was measured the by MTT. As shown in Fig. 3B, BAPTA-AM could significantly inhibit the surfactin-induced apoptosis (p < 0.01). These data suggested [Ca2+]i exaltation induced cells apoptosis (Fig. 3).
Rearrangement of [Ca2+]i
It is well known that ER is crucial to [Ca2+]i homeostasis, the [Ca2+]i level is partly modulated by activation of IP3 receptors (IP3Rs) and activation of ryanodine receptors (RyRs) on the ER [9]. Heparin and ruthenium red were used as effective competitive antagonist of IP3Rs and RyRs. As shown in Fig. 4, Ca2+ was distributed evenly in the surfactin group compared with control and the Ca2+ was mostly locked in certain area in the cells pretreated with heparin or ruthenium red compared with surfactin group. It indicated that [Ca2+]i exaltation was partly depended on Ca2+ release mediated by IP3Rs and RyRs (Fig. 4).
Rearrangement of [Ca2+]i in surfactin-induced HepG2 cells. A Observation Ca2+ rearrangement through IP3Rs. a Control. b HepG2 cells were incubated with 10 mg/ml heparin. c 10 mg/ml heparin was added into HepG2 cells for 2 h, and then incubated with surfactin (42 μg/ml) for 9 h. d HepG2 cells were incubated with 42 μg/ml surfactin for 9 h. B Observation Ca2+ rearrangement through RyRs. a Control. b HepG2 cells were incubated with surfactin (42 μg/ml) for 9 h. c 5 μM Ruth was added into HepG2 cells for 2 h, and then incubated with surfactin (42 μg/ml) for 9 h. d HepG2 cells were incubated with 42 μg/ml surfactin for 9 h
Testing ERS Induced by Ca2+ Rearrangement
ERS occurred in ER, upregulated CHOP/Gadd153 expression is a critical cellular response for ERS-induced apoptosis. Hence, the CHOP/Gadd153 expression was detected to represent ERS level. As shown in Fig. 5a, b, surfactin triggered CHOP/Gadd153 expression, and the CHOP/Gadd153 expression was reduced remarkably in pretreated with heparin or ruthenium red compared to surfactin group (p < 0.01), suggesting that Ca2+ release both mediated by IP3 and Ry receptors triggered ERS. Surfactin-induced [Ca2+]i exaltation which was depended on Ca2+ release mediated by IP3 and Ry receptors (Fig. 5).
Testing ERS induced by Ca2+ rearrangement. A Observation the ERS on Ca2+ release from IP3 channel by western blotting. B Observation the ERS on Ca2+ release from Ry channel by western blotting. C Quantification of effect of heparin on CHOP expression in HepG2 cells. D Quantification of effect of Ruth on CHOP expression in HepG2 cells. **p < 0.01 compared with surfactin group
Relationship Between ROS and [Ca2+]i
Our results showed that both [Ca2+]i increase and ROS generation were involved in surfactin-induced apoptosis. To further determine the relationship between ROS generation and the increase of [Ca2+]i, cells were, respectively, pretreated with NAC or BAPTA-AM. As shown in Fig. 6, the use of NAC to chelate ROS successfully prevented [Ca2+]i, however, BAPTA-AM had no obvious effect on ROS generation, it seemed that [Ca2+]i increase was dependent on ROS generation. This leads us to hypothesize that ROS may act as an upstream regulator of surfactin-induced [Ca2+]i (Fig. 6).
Relationship between ROS and Ca2+. A The effect of BAPTA-AM (the chelator of calcium) on intracellular ROS production. a HepG2 cells were incubated with surfactin (42 μg/ml) for 0 h. b HepG2 cells were incubated with surfactin (42 μg/ml) for 4 h. c 1 mM NAC was added into HepG2 cells for 2 h and then incubated with surfactin for 4 h. B The effect of NAC (the chelator of ROS) on [Ca2+]i. a HepG2 cells were incubated with surfactin (42 μg/ml) for 0 h. b HepG2 cells were incubated with surfactin (42 μg/ml) for 9 h. c 1 mM NAC was added into HepG2 cells for 2 h and then incubated with surfactin for 9 h
Roles of MAPK Signaling in Surfactin-Induced Apoptosis
The MAPK signaling pathways are activated by different extracellular stimuli, resulting in a wide range of cellular responses, including apoptosis and proliferation [7]. Hence, the expression of the phosphorylated forms of ERK1/2, p38-mitogen-activated protein kinases (p38), and c-Jun N-terminal kinase (JNK) were evaluated. As shown in Fig. 7, the expression of p-ERK was reduced as a time manner, but no obvious changes on phosphorylated forms of p38 and JNK, suggesting that blocking ERK1/2 pathway was specifically linked to surfactin-induced HepG2 cells apoptosis (Fig. 7).
Relationship Between ERK Pathway and [Ca2+]i
To further determine the relationship between extracellular signal-regulated kinase (ERK) pathway and [Ca2+]i, cells were, respectively, pretreated with PD98059 (inhibitor of ERK) or BAPTA-AM. As shown in Fig. 8, the use of BAPTA-AM to inhibit [Ca2+]i successfully increased [Ca2+]i the level of p-ERK (p < 0.01), however, PD98059 had no obvious effect on [Ca2+]i. It suggested that blocking ERK pathway was regulated by [Ca2+]i level in surfactin-induced apoptosis (Fig. 8).
Relationship between ERK pathway and [Ca2+]i. A Observation the effect of ERK pathway on Ca2+ level by fluorescence microscopy. a HepG2 cells were incubated with surfactin (42 μg/ml) for 0 h. b HepG2 cells were incubated with surfactin (42 μg/ml) for 9 h. c HepG2 cells were incubated with 10 μM PD98059 for 9 h. d 10 μM PD98059 was added into HepG2 cells for 2 h, then incubated with surfactin (42 μg/ml) for 9 h. B Testing the impact of the Ca2+ level in MAPK pathways by western blotting. C Quantification of effect of BAPTA on P-ERK expression in HepG2 cells. **p < 0.01 compared with surfactin group
Discussion
Surfactin has been known to inhibit proliferation and induce apoptosis in cancer cells [10], however, the molecular mechanism(s) responsible for these processes remain elusive. In this study, it has clearly certificated that ROS production and Ca2+ levels could cause apoptosis. ER stress hallmark Bip/GRP78 and CHOP/Gadd153 expression increased and the NAC inhibited the expression of the two proteins. It was clear that ERS was regulated by ROS production.
Disruption of cellular Ca2+ homoeostasis and the release of Ca2+ from intracellular storage into the cytosol is central to apoptosis. The results suggested that the increase of [Ca2+]i was depended on the IP3 and Ry receptors channels. Then, the ERS was tested after being blocked the IP3 and Ry receptors channels. The results illustrated that the ERS was triggered by surfactin, and regulated by Ca2+ released from IP3 and Ry receptors channels.
The MAPK family respond to extracellular stimuli such as proliferation and cell apoptosis [13]. The results indicated that surfactin-induced HepG2 cells apoptosis was involved in down-regulated ERK1/2, and blocking of ERK1/2 pathway was regulated by ROS-mediated [Ca2+]i level.
In summary, our results showed that surfactin-induced apoptosis is mediated by ROS-activated ERS, resulted in Ca2+ release, which could also strengthen ERS, followed by ERK blocking in HepG2 cells. Further studies will focus on the feedback of ER to ROS and Ca2+, analysis of mitochondrial Ca2+ concentration, and relationship between mitochondria and ER.
References
Aizawa, Y. (2011). Sex differences play a role in cardiac endoplasmic reticulum stress (ERS) and ERS-initiated apoptosis induced by pressure overload and thapsigargin. Cardiovascular Pathology, 20, 281–290.
Breitwieser, G. E. (2006). Calcium sensing receptors and calcium oscillations: Calcium as a first messenger. Current Topics in Developmental Biology, 73, 85–114.
Burlacu, A. (2003). Regulation of apoptosis by Bcl-2 family proteins. Journal of Cellular and Molecular Medicine, 7, 249–257.
Cao, X. H., Wang, A. H., Jiao, R. Z., Wang, C. L., Mao, D. Z., & Lu, M. F. (2010). Surfactin induces apoptosis in human breast cancer MCF-7 cells through a ROS/JNK-mediated mitochondrial/caspase pathway. Chemico-Biological Interactions, 183, 357–362.
Cory, S., Huang, D. C. S., & Adams, J. M. (2003). The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene, 22, 8590–8607.
Deng, X., Yin, F., Lu, X., Cai, B., & Yin, W. (2006). The apoptotic effect of brucine from the seed of strychnos nux-vomica on human hepatoma cells is mediated via Bcl-2 and Ca2+ involved mitochondrial pathway. Toxicological Sciences, 91, 59–69.
Diffley, J. M., Wu, M., Sohn, M., Song, W., Hammad, S. M., & Lyons, T. J. (2009). Apoptosis induction by oxidized glycated LDL in human retinal capillary pericytes is independent of activation of MAPK signaling pathways. Molecular Vision, 15, 135–145.
Hajnoczky, G., Davies, E., & Madesh, M. (2003). Calcium signaling and apoptosis. Biochemical and Biophysical Research Communications, 304, 445–454.
Hao, L., Zhang, Q., Yu, T., Yu, L., & Cheng, Y. (2011). Modulation of ultra low molecular weight heparin on [Ca2+]i in nervous cells. Brain Research Bulletin, 86, 355–359.
Kameda, Y., Oira, S., Matsui, K., Kanatomo, S., & Hase, T. (1974). Anti-tumor activity of Bacillus natto. V. isolation and characterization of surfactin in the culture medium of Bacillus natto KMD 2311. Chemical & Pharmaceutical Bulletin, 22, 938–944.
Leber, B., Lin, J., & Andrews, D. W. (2007). Embedded together: the life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis, 12, 897–911.
Nakano, H., Nakajima, A., Sakon, S., Piao, J. H., Xue, X., & Okumura, K. (2006). Reactive oxygen species mediate crosstalk between NF-kappaB and JNK. Cell Death and Differentiation, 13, 730–737.
Pearson, G., Robinson, F., Gibson, T. B., Xu, B., Karandikar, M., Berman, K., et al. (2001). Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocrine Reviews, 22, 153–183.
Peypoux, F., Bonmatin, J. M., & Wallach, J. (1999). Recent trends in the biochemistry of surfactin. Applied Microbiology and Biotechnology, 51, 553–563.
Sari, F. R., Watanabe, K., Widyantoro, B., Thandavarayan, R. A., Harima, M., Kodama, M., et al. (2004). Potential applications of microbial surfactants in biomedical sciences. Trends in Biotechnology, 22, 142–146.
Sergeev, I. N. (2005). Calcium signaling in cancer and vitamin D. Journal of Steroid Biochemistry and molecular Biology, 97, 145–151.
Shen, H. M., & Liu, Z. G. (2006). JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radical Biology and Medicine, 40, 928–939.
Temkin, V., & Karin, M. (2007). From death receptor to reactive oxygen species and c-Jun N-terminal protein kinase: The receptor-interacting protein 1 odyssey. Immunological Reviews, 220, 8–21.
Wang, C. L., Ng, T. B., Cao, X. H., Jiang, Y., Liu, Z. K., Wen, T. Y., et al. (2009). CLP induces apoptosis in K562 cells through Ca2+ regulating ERK activation. Cancer Letters, 276, 221–227.
Ye, J. L., Mao, W. P., Wu, A. L., Zhang, N. N., Zhou, L., & Wei, C. J. (2007). Cadmium-induced apoptosis in human normal liver L-02 cells by acting on mitochondria and regulating Ca2+ signals. Environmental Toxicology and Pharmacology, 24, 45–50.
Acknowledgments
This study was supported by the National Nature Science Foundation of China (31000768), the plan for the development of Changjiang Scholars and innovative research team (IRT1166) and the Key Projects in National Science & Technology Pillar Program during the 12th Five-Year Plan Period (2012BAD33B04).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Wang, Cl., Liu, C., Niu, Ll. et al. Surfactin-Induced Apoptosis Through ROS–ERS–Ca2+-ERK Pathways in HepG2 Cells. Cell Biochem Biophys 67, 1433–1439 (2013). https://doi.org/10.1007/s12013-013-9676-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9676-7