Abstract
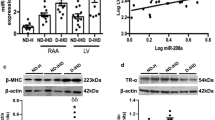
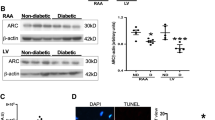
Diabetic cardiomyopathy is a leading cause of morbidity and mortality, and Insulin2 mutant (Ins2+/−) Akita is a genetic mice model of diabetes relevant to humans. Dicer, miRNAs, and inflammatory cytokines are associated with heart failure. However, the differential expression of miRNAs, dicer, and inflammatory molecules are not clear in diabetic cardiomyopathy of Akita. We measured the levels of miRNAs, dicer, pro-inflammatory tumor necrosis factor alpha (TNFα), and anti-inflammatory interleukin 10 (IL-10) in C57BL/6J (WT) and Akita hearts. The results revealed increased heart to body weight ratio and robust expression of brain natriuretic peptide (BNP: a hypertrophy marker) suggesting cardiac hypertrophy in Akita. The multiplex RT-PCR, qPCR, and immunoblotting showed up regulation of dicer, whereas miRNA array elicited spread down regulation of miRNAs in Akita including dramatic down regulation of let-7a, miR-130, miR-142-3p, miR-148, miR-338, miR-345-3p, miR-384-3p, miR-433, miR-450, miR-451, miR-455, miR-494, miR-499, miR-500, miR-542-3p, miR-744, and miR-872. Conversely, miR-295 is induced in Akita. Cardiac TNFα is upregulated at mRNA (RT-PCR and qPCR), protein (immunoblotting), and cellular (immunohistochemistry and confocal microscopy) levels, and is robust in hypertrophic cardiomyocytes suggesting direct association of TNFα with hypertrophy. Contrary to TNFα, cardiac IL-10 is downregulated in Akita. In conclusion, induction of dicer and TNFα, and attenuation of IL-10 and majority of miRNA are associated with cardiomyopathy in Akita and could be used for putative therapeutic target for heart failure in diabetics.





Similar content being viewed by others
References
Garin, I., Edghill, E. L., Akerman, I., Rubio-Cabezas, O., Rica, I., Locke, J. M., et al. (2010). Recessive mutations in the INS gene result in neonatal diabetes through reduced insulin biosynthesis. Proceedings of the National Academy of Sciences of the United States of America, 107(7), 3105–3110.
Barber, A. J., Antonetti, D. A., Kern, T. S., Reiter, C. E., Soans, R. S., Krady, J. K., et al. (2005). The Ins2Akita mouse as a model of early retinal complications in diabetes. Investigative Ophthalmology and Visual Science, 46(6), 2210–2218.
Chang, J. H., Paik, S. Y., Mao, L., Eisner, W., Flannery, P. J., Wang, L., et al. (2012). Diabetic kidney disease in FVB/NJ Akita mice: Temporal pattern of kidney injury and urinary nephrin excretion. PLoS ONE, 7(4), e33942.
Mishra, P. K., Givvimani, S., Metreveli, N., & Tyagi, S. C. (2010). Attenuation of beta2-adrenergic receptors and homocysteine metabolic enzymes cause diabetic cardiomyopathy. Biochemical and Biophysical Research Communications, 401(2), 175–181.
Mishra, P. K., Tyagi, N., Sen, U., Joshua, I. G., & Tyagi, S. C. (2010). Synergism in hyperhomocysteinemia and diabetes: Role of PPAR gamma and tempol. Cardiovascular Diabetology, 9, 49.
Izumi, T., Yokota-Hashimoto, H., Zhao, S., Wang, J., Halban, P. A., & Takeuchi, T. (2003). Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes, 52(2), 409–416.
Wang, J., Takeuchi, T., Tanaka, S., Kubo, S. K., Kayo, T., Lu, D., et al. (1999). A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. Journal of Clinical Investigation, 103(1), 27–37.
Hartemann, A., & Bourron, O. (2012). Interleukin-2 and type 1 diabetes: New therapeutic perspectives. Diabetes and Metabolism, 38(5), 387–391.
Epstein, P. N., Overbeek, P. A., & Means, A. R. (1989). Calmodulin-induced early-onset diabetes in transgenic mice. Cell, 58(6), 1067–1073.
Li, Y., Hamasaki, T., Teruya, K., Nakamichi, N., Gadek, Z., Kashiwagi, T., et al. (2012). Suppressive effects of natural reduced waters on alloxan-induced apoptosis and type 1 diabetes mellitus. Cytotechnology, 64(3), 281–297.
Li, Y. Y., Liu, H. H., Chen, H. L., & Li, Y. P. (2012). Adipose-derived mesenchymal stem cells ameliorate STZ-induced pancreas damage in type 1 diabetes. BioMedical Materials and Engineering, 22(1), 97–103.
Yaghmaei, P., Esfahani-Nejad, H., Ahmadi, R., Hayati-Roodbari, N., & Ebrahim-Habibi, A. (2012). Maternal zinc intake of Wistar rats has a protective effect in the alloxan-induced diabetic offspring. Journal of Physiology and Biochemistry, 69(1), 35–43.
Mishra, P. K., Chavali, V., Metreveli, N., & Tyagi, S. C. (2012). Ablation of MMP9 induces survival and differentiation of cardiac stem cells into cardiomyocytes in the heart of diabetics: A role of extracellular matrix. Canadian Journal of Physiology and Pharmacology, 90(3), 353–360.
Patel, V. B., Bodiga, S., Basu, R., Das, S. K., Wang, W., Wang, Z., et al. (2012). Loss of angiotensin-converting enzyme-2 exacerbates diabetic cardiovascular complications and leads to systolic and vascular dysfunction: A critical role of the angiotensin II/AT1 receptor axis. Circulation Research, 110(10), 1322–1335.
Bartel, D. P. (2004). MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell, 116(2), 281–297.
Bartel, D. P. (2009). MicroRNAs: Target recognition and regulatory functions. Cell, 136(2), 215–233.
Kawashima, T., & Shioi, T. (2011). MicroRNA, emerging role as a biomarker of heart failure. Circulation Journal, 75(2), 268–269.
Mishra, P. K., Tyagi, N., Kumar, M., & Tyagi, S. C. (2009). MicroRNAs as a therapeutic target for cardiovascular diseases. Journal of Cellular and Molecular Medicine, 13(4), 778–789.
Ono, K., Kuwabara, Y., & Han, J. (2011). MicroRNAs and cardiovascular diseases. FEBS Journal, 278(10), 1619–1633.
Papageorgiou, N., Tousoulis, D., Androulakis, E., Siasos, G., Briasoulis, A., Vogiatzi, G., et al. (2012). The role of microRNAs in cardiovascular disease. Current Medicinal Chemistry, 19(16), 2605–2610.
Sayed, D., Hong, C., Chen, I. Y., Lypowy, J., & Abdellatif, M. (2007). MicroRNAs play an essential role in the development of cardiac hypertrophy. Circulation Research, 100(3), 416–424.
van Rooij, E., Sutherland, L. B., Liu, N., Williams, A. H., McAnally, J., Gerard, R. D., et al. (2006). A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proceedings of the National Academy of Sciences of the United States of America, 103(48), 18255–18260.
Greco, S., Fasanaro, P., Castelvecchio, S., D’Alessandra, Y., Arcelli, D., Di, D. M., et al. (2012). MicroRNA dysregulation in diabetic ischemic heart failure patients. Diabetes, 61(6), 1633–1641.
Guay, C., Roggli, E., Nesca, V., Jacovetti, C., & Regazzi, R. (2011). Diabetes mellitus, a microRNA-related disease? Translational Research, 157(4), 253–264.
Kantharidis, P., Wang, B., Carew, R. M., & Lan, H. Y. (2011). Diabetes complications: The microRNA perspective. Diabetes, 60(7), 1832–1837.
Tyagi, A. C., Sen, U., & Mishra, P. K. (2011). Synergy of microRNA and stem cell: A novel therapeutic approach for diabetes mellitus and cardiovascular diseases. Current Diabetes Review, 7(6), 367–376.
Care, A., Catalucci, D., Felicetti, F., Bonci, D., Addario, A., Gallo, P., et al. (2007). MicroRNA-133 controls cardiac hypertrophy. Nature Medicine, 13(5), 613–618.
Feng, B., Chen, S., George, B., Feng, Q., & Chakrabarti, S. (2010). miR133a regulates cardiomyocyte hypertrophy in diabetes. Diabetes/Metabolism: Research and Reviews, 26(1), 40–49.
Belevych, A. E., Sansom, S. E., Terentyeva, R., Ho, H. T., Nishijima, Y., Martin, M. M., et al. (2011). MicroRNA-1 and -133 increase arrhythmogenesis in heart failure by dissociating phosphatase activity from RyR2 complex. PLoS ONE, 6(12), e28324.
Luo, X., Lin, H., Pan, Z., Xiao, J., Zhang, Y., Lu, Y., et al. (2008). Down-regulation of miR-1/miR-133 contributes to re-expression of pacemaker channel genes HCN2 and HCN4 in hypertrophic heart. Journal of Biological Chemistry, 283(29), 20045–20052.
Xiao, J., Luo, X., Lin, H., Zhang, Y., Lu, Y., Wang, N., et al. (2007). MicroRNA miR-133 represses HERG K+ channel expression contributing to QT prolongation in diabetic hearts. Journal of Biological Chemistry, 282(17), 12363–12367.
Castoldi, G., Di Gioia, C. R., Bombardi, C., Catalucci, D., Corradi, B., Gualazzi, M. G., et al. (2012). MiR-133a regulates collagen 1A1: Potential role of miR-133a in myocardial fibrosis in angiotensin II-dependent hypertension. Journal of Cellular Physiology, 227(2), 850–856.
Matkovich, S. J., Wang, W., Tu, Y., Eschenbacher, W. H., LE Dorn, Condorelli, G., et al. (2010). MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circulation Research, 106(1), 166–175.
Chavali, V., Tyagi, S. C., & Mishra, P. K. (2012). MicroRNA-133a regulates DNA methylation in diabetic cardiomyocytes. Biochemical and Biophysical Research Communications, 425(3), 668–672.
Bernstein, E., Kim, S. Y., Carmell, M. A., Murchison, E. P., Alcorn, H., Li, M. Z., et al. (2003). Dicer is essential for mouse development. Nature Genetics, 35(3), 215–217.
Davis, T. H., Cuellar, T. L., Koch, S. M., Barker, A. J., Harfe, B. D., McManus, M. T., et al. (2008). Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. Journal of Neuroscience, 28(17), 4322–4330.
Koralov, S. B., Muljo, S. A., Galler, G. R., Krek, A., Chakraborty, T., Kanellopoulou, C., et al. (2008). Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell, 132(5), 860–874.
Kuehbacher, A., Urbich, C., Zeiher, A. M., & Dimmeler, S. (2007). Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circulation Research, 101(1), 59–68.
Lynn, F. C., Skewes-Cox, P., Kosaka, Y., McManus, M. T., Harfe, B. D., & German, M. S. (2007). MicroRNA expression is required for pancreatic islet cell genesis in the mouse. Diabetes, 56(12), 2938–2945.
Murchison, E. P., Stein, P., Xuan, Z., Pan, H., Zhang, M. Q., Schultz, R. M., et al. (2007). Critical roles for Dicer in the female germline. Genes and Development, 21(6), 682–693.
Zhao, Y., Ransom, J. F., Li, A., Vedantham, V., von, D. M., Muth, A. N., et al. (2007). Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell, 129(2), 303–317.
Chen, J. F., Murchison, E. P., Tang, R., Callis, T. E., Tatsuguchi, M., Deng, Z., et al. (2008). Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proceedings of the National Academy of Sciences of the United States of America, 105(6), 2111–2116.
Da Costa Martins, P. A., Bourajjaj, M., Gladka, M., Kortland, M., van Oort, R. J., Pinto, Y. M., et al. (2008). Conditional dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation, 118(15), 1567–1576.
Tokumaru, S., Suzuki, M., Yamada, H., Nagino, M., & Takahashi, T. (2008). let-7 regulates Dicer expression and constitutes a negative feedback loop. Carcinogenesis, 29(11), 2073–2077.
Martello, G., Rosato, A., Ferrari, F., Manfrin, A., Cordenonsi, M., Dupont, S., et al. (2010). A MicroRNA targeting dicer for metastasis control. Cell, 141(7), 1195–1207.
Moschos, S. A., Williams, A. E., Perry, M. M., Birrell, M. A., Belvisi, M. G., & Lindsay, M. A. (2007). Expression profiling in vivo demonstrates rapid changes in lung microRNA levels following lipopolysaccharide-induced inflammation but not in the anti-inflammatory action of glucocorticoids. BMC Genomics, 8, 240.
Perry, M. M., Moschos, S. A., Williams, A. E., Shepherd, N. J., Larner-Svensson, H. M., & Lindsay, M. A. (2008). Rapid changes in microRNA-146a expression negatively regulate the IL-1beta-induced inflammatory response in human lung alveolar epithelial cells. Journal of Immunology, 180(8), 5689–5698.
Roy, S., & Sen, C. K. (2011). miRNA in innate immune responses: Novel players in wound inflammation. Physiological Genomics, 43(10), 557–565.
Wang, J. F., Yu, M. L., Yu, G., Bian, J. J., Deng, X. M., Wan, X. J., et al. (2010). Serum miR-146a and miR-223 as potential new biomarkers for sepsis. Biochemical and Biophysical Research Communications, 394(1), 184–188.
Zeng, J. R., Xu, X. L., Yu, X. J., Hou, J., Xu, T. J., Mi, M., et al. (2012). Dynamic correlation of TNF-alpha and IL-10 with myocardial remodeling induced by pressure overload in rats. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi, 28(7), 699–701.
Cain, B. S., Meldrum, D. R., Dinarello, C. A., Meng, X., Joo, K. S., Banerjee, A., et al. (1999). Tumor necrosis factor-alpha and interleukin-1beta synergistically depress human myocardial function. Critical Care Medicine, 27(7), 1309–1318.
Bozkurt, B., Kribbs, S. B., Clubb, F. J, Jr, Michael, L. H., Didenko, V. V., Hornsby, P. J., et al. (1998). Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation, 97(14), 1382–1391.
Calle, M. C., & Fernandez, M. L. (2012). Inflammation and type 2 diabetes. Diabetes and Metabolism, 38(3), 183–191.
Bradham, W. S., Bozkurt, B., Gunasinghe, H., Mann, D., & Spinale, F. G. (2002). Tumor necrosis factor-alpha and myocardial remodeling in progression of heart failure: A current perspective. Cardiovascular Research, 53(4), 822–830.
Bradham, W. S., Moe, G., Wendt, K. A., Scott, A. A., Konig, A., Romanova, M., et al. (2002). TNF-alpha and myocardial matrix metalloproteinases in heart failure: Relationship to LV remodeling. American Journal of Physiology: Heart and Circulatory Physiology, 282(4), H1288–H1295.
Dhingra, S., Bagchi, A. K., Ludke, A. L., Sharma, A. K., & Singal, P. K. (2011). Akt regulates IL-10 mediated suppression of TNFalpha-induced cardiomyocyte apoptosis by upregulating Stat3 phosphorylation. PLoS ONE, 6(9), e25009.
Krishnamurthy, P., Rajasingh, J., Lambers, E., Qin, G., Losordo, D. W., & Kishore, R. (2009). IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circulation Research, 104(2), e9–e18.
Verma, S. K., Krishnamurthy, P., Barefield, D., Singh, N., Gupta, R., Lambers, E., et al. (2012). Interleukin-10 treatment attenuates pressure overload-induced hypertrophic remodeling and improves heart function via signal transducers and activators of transcription 3-dependent inhibition of nuclear factor-kappaB. Circulation, 126(4), 418–429.
Barac, A., Wang, H., Shara, N. M., Simone, G., Carter, E. A., Umans, J. G., et al. (2012). Markers of inflammation, metabolic risk factors, and incident heart failure in American Indians: The Strong Heart Study. Journal of Clinical Hypertension (Greenwich), 14(1), 13–19.
Mishra, P. K., Tyagi, N., Kundu, S., & Tyagi, S. C. (2009). MicroRNAs are involved in homocysteine-induced cardiac remodeling. Cell Biochemistry and Biophysics, 55(3), 153–162.
Mishra, P. K., Awe, O., Metreveli, N., Qipshidze, N., Joshua, I. G., & Tyagi, S. C. (2011). Exercise mitigates homocysteine—beta2-adrenergic receptor interactions to ameliorate contractile dysfunction in diabetes. International Journal of Physiology, Pathophysiology and Pharmacology, 3(2), 97–106.
Salgo, I. S., Tsang, W., Ackerman, W., Ahmad, H., Chandra, S., Cardinale, M., et al. (2012). Geometric assessment of regional left ventricular remodeling by three-dimensional echocardiographic shape analysis correlates with left ventricular function. Journal of the American Society of Echocardiography, 25(1), 80–88.
Schaefer, J. S., Montufar-Solis, D., Vigneswaran, N., & Klein, J. R. (2011). Selective upregulation of microRNA expression in peripheral blood leukocytes in IL-10-/- mice precedes expression in the colon. Journal of Immunology, 187(11), 5834–5841.
van de Vrie, M., Heymans, S., & Schroen, B. (2011). MicroRNA involvement in immune activation during heart failure. Cardiovascular Drugs and Therapy, 2, 161–170.
Roger, V. L., Go, A. S., Lloyd-Jones, D. M., Benjamin, E. J., Berry, J. D., Borden, W. B., et al. (2012). Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation, 125(1), e2–e220.
Pignone, M., Alberts, M. J., Colwell, J. A., Cushman, M., Inzucchi, S. E., Mukherjee, D., et al. (2010). Aspirin for primary prevention of cardiovascular events in people with diabetes: A position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care, 33(6), 1395–1402.
King, H., Aubert, R. E., & Herman, W. H. (1998). Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care, 21(9), 1414–1431.
Wild, S., Roglic, G., Green, A., Sicree, R., & King, H. (2004). Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care, 27(5), 1047–1053.
Shantikumar, S., Caporali, A., & Emanueli, C. (2012). Role of microRNAs in diabetes and its cardiovascular complications. Cardiovascular Research, 93(4), 583–593.
Huang, Y., Crawford, M., Higuita-Castro, N., Nana-Sinkam, P., & Ghadiali, S. N. (2012). miR-146a regulates mechanotransduction and pressure-induced inflammation in small airway epithelium. FASEB Journal, 26(8), 3351–3364.
Zidar, N., Bostjancic, E., Glavac, D., & Stajer, D. (2011). MicroRNAs, innate immunity and ventricular rupture in human myocardial infarction. Disease Markers, 31(5), 259–265.
Manabe, I. (2011). Chronic inflammation links cardiovascular, metabolic and renal diseases. Circulation Journal, 75(12), 2739–2748.
Rosner, M. H., Ronco, C., & Okusa, M. D. (2012). The role of inflammation in the cardio-renal syndrome: A focus on cytokines and inflammatory mediators. Seminars in Nephrology, 32(1), 70–78.
Monsefi, N., Zierer, A., Bakhtiary, F., Vogl, T., Ackermann, H., Kleine, P., et al. (2012). Spherical dilatation of the apex in failing left ventricles: A target for surgical remodelling techniques. Journal of Cardiovascular Surgery (Torino), 53(4), 545–552.
Basu, R., Oudit, G. Y., Wang, X., Zhang, L., Ussher, J. R., Lopaschuk, G. D., et al. (2009). Type 1 diabetic cardiomyopathy in the Akita (Ins2WT/C96Y) mouse model is characterized by lipotoxicity and diastolic dysfunction with preserved systolic function. American Journal of Physiology: Heart and Circulatory Physiology, 297(6), H2096–H2108.
Staszel, T., Zapala, B., Polus, A., Sadakierska-Chudy, A., Kiec-Wilk, B., Stepien, E., et al. (2011). Role of microRNAs in endothelial cell pathophysiology. Polskie Archiwum Medycyny Wewnetrznej, 121(10), 361–366.
Duisters, R. F., Tijsen, A. J., Schroen, B., Leenders, J. J., Lentink, V., van der Made, I., et al. (2009). miR-133 and miR-30 regulate connective tissue growth factor: Implications for a role of microRNAs in myocardial matrix remodeling. Circulation Research, 104(2), 170–178.
Medeiros, L. A., Dennis, L. M., Gill, M. E., Houbaviy, H., Markoulaki, S., Fu, D., et al. (2011). Mir-290-295 deficiency in mice results in partially penetrant embryonic lethality and germ cell defects. Proceedings of the National Academy of Sciences of the United States of America, 108(34), 14163–14168.
Thum, T., Galuppo, P., Wolf, C., Fiedler, J., Kneitz, S., van Laake, L. W., et al. (2007). MicroRNAs in the human heart: A clue to fetal gene reprogramming in heart failure. Circulation, 116(3), 258–267.
Johnnidis, J. B., Harris, M. H., Wheeler, R. T., Stehling-Sun, S., Lam, M. H., Kirak, O., et al. (2008). Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature, 451(7182), 1125–1129.
Chao, W. (2009). Toll-like receptor signaling: A critical modulator of cell survival and ischemic injury in the heart. American Journal of Physiology: Heart and Circulatory Physiology, 296(1), H1–H12.
Mann, D. L., Topkara, V. K., Evans, S., & Barger, P. M. (2010). Innate immunity in the adult mammalian heart: For whom the cell tolls. Transactions of the American Clinical and Climatological Association, 121, 34–50.
Garlie, J. B., Hamid, T., Gu, Y., Ismahil, M. A., Chandrasekar, B., & Prabhu, S. D. (2011). Tumor necrosis factor receptor 2 signaling limits beta-adrenergic receptor-mediated cardiac hypertrophy in vivo. Basic Research in Cardiology, 106(6), 1193–1205.
Acknowledgments
The financial supports from American Heart Association Grant (11BGIA 7690055) and National Institute of Health (HL-113281) to P.K.M. and National Institute of Health (HL-108621 and HL-74185) to S.C.T. is gratefully acknowledged.
Conflict of interest
Authors confirm that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chavali, V., Tyagi, S.C. & Mishra, P.K. Differential Expression of Dicer, miRNAs, and Inflammatory Markers in Diabetic Ins2+/− Akita Hearts. Cell Biochem Biophys 68, 25–35 (2014). https://doi.org/10.1007/s12013-013-9679-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9679-4