Abstract
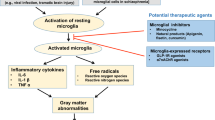
Activation of microglia and inflammation-mediated neurotoxicity are believed to play an important role in the pathogenesis of several neurodegenerative disorders, including multiple sclerosis. Studies demonstrate complex functions of activated microglia that can lead to either beneficial or detrimental outcomes, depending on the form and the timing of activation. Combined with genetic and environmental factors, overactivation and dysregulation of microglia cause progressive neurotoxic consequences which involve a vicious cycle of neuron injury and unregulated neuroinflammation. Thus, modulation of microglial activation appears to be a promising new therapeutic target. While current therapies do attempt to block activation of microglia, they indiscriminately inhibit inflammation thus also curbing beneficial effects of inflammation and delaying recovery. Multiple signaling cascades, often cross-talking, are involved in every step of microglial activation. One of the key challenges is to understand the molecular mechanisms controlling cytokine expression and phagocytic activity, as well as cell-specific consequences of dysregulated cytokine expression. Further, a better understanding of how the integration of multiple cytokine signals influences the function or activity of individual microglia remains an important research objective to identify potential therapeutic targets for clinical intervention to promote repair.



Similar content being viewed by others
References
Campbell, A. (2004). Inflammation, neurodegenerative diseases, and environmental exposures. Annals of the New York Academy of Sciences, 1035, 117–132.
Rivest, S. (2003). Molecular insights on the cerebral innate immune system. Brain, Behavior, and Immunity, 17, 13–19.
Nadeau, S., & Rivest, S. (2003). Glucocorticoids play a fundamental role in protecting the brain during innate immune response. Journal of Neuroscience, 23, 5536–5544.
Gao, Z., & Tsirka, S. E. (2011). Animal models of MS reveal multiple roles of microglia in disease pathogenesis. Neurology Research International, 2011, 383087.
Barnett, M. H., & Prineas, J. W. (2004). Relapsing and remitting multiple sclerosis: Pathology of the newly forming lesion. Annals of Neurology, 55, 458–468.
Barnett, M. H., Henderson, A. P., & Prineas, J. W. (2006). The macrophage in MS: Just a scavenger after all? Pathology and pathogenesis of the acute MS lesion. Multiple Sclerosis, 12, 121–132.
Bertram, L., & Tanzi, R. E. (2005). The genetic epidemiology of neurodegenerative disease. The Journal of Clinical Investigation, 115, 1449–1457.
Griffin, W. S. (2006). Inflammation and neurodegenerative diseases. American Journal of Clinical Nutrition, 2006(83), 470S–474S.
Keen, D., & Ward, S. (2004). Autistic spectrum disorder: A child population profile. Autism, 8, 39–48.
Kern, J. K., & Jones, A. M. (2006). Evidence of toxicity, oxidative stress, and neuronal insult in autism. Journal of Toxicology and Environmental Health, Part B. Critical Reviews, 9, 485–499.
Larsson, H. J., Eaton, W. W., Madsen, K. M., Vestergaard, M., Olesen, A. V., Agerbo, E., et al. (2005). Risk factors for autism: Perinatal factors, parental psychiatric history, and socioeconomic status. American Journal of Epidemiology, 161, 916–925.
Muravchick, S., & Levy, R. J. (2006). Clinical implications of mitochondrial dysfunction. Anesthesiology, 105, 819–837.
Granieri, E., Casetta, I., Tola, M. R., & Ferrante, P. (2001). Multiple sclerosis: Infectious hypothesis. Neurological Sciences, 22, 179–185.
Hauss-Wegrzyniak, B., Dobrzanski, P., Stoehr, J. D., & Wenk, G. L. (1998). Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer’s disease. Brain Research, 780, 294–303.
Calderon-Garciduenas, L., Azzarelli, B., Acuna, H., Garcia, R., Gambling, T. M., Osnaya, N., et al. (2002). Air pollution and brain damage. Toxicologic Pathology, 30, 373–389.
Authier, F. J., Cherin, P., Creange, A., Bonnotte, B., Ferrer, X., Abdelmoumni, A., et al. (2001). Central nervous system disease in patients with macrophagic myofasciitis. Brain, 124, 974–983.
Bishop, N. J., Morley, R., Day, J. P., & Lucas, A. (1997). Aluminum neurotoxicity in preterm infants receiving intravenous-feeding solutions. New England Journal of Medicine, 336, 1557–1561.
Ngoi, S. M., Sylvester, F. A., & Vella, A. T. (2011). The role of microbial byproducts in protection against immunological disorders and the hygiene hypothesis. Discovery Medicines, 12, 405–412.
Schulte, C., & Gasser, T. (2011). Genetic basis of Parkinson’s disease: Inheritance, penetrance, and expression. Application of Clinical Genetics, 4, 80.
Scrimshaw, N. S., & SanGiovanni, J. P. (1997). Synergism of nutrition, infection, and immunity: An overview. American Journal of Clinical Nutrition, 66, 464S–477S.
Tollervey, J. R., Wang, Z., Hortobagyi, T., Witten, J. T., Zarnack, K., Kayikci, M., et al. (2011). Analysis of alternative splicing associated with aging and neurodegeneration in the human brain. Genome Research, 21, 1572–1582.
Glovsky, M. M., Ward, P. A., & Johnson, K. J. (2004). Complement determinations in human disease. Annals of Allergy, Asthma & Immunology, 93, 513–522.
Gao, H. M., Zhou, H., Zhang, F., Wilson, B. C., Kam, W., & Hong, J. S. (2011). HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. Journal of Neuroscience, 31, 1081–1092.
Pei, Z., Pang, H., Qian, L., Yang, S., Wang, T., Zhang, W., et al. (2007). MAC1 mediates LPS-induced production of superoxide by microglia: The role of pattern recognition receptors in dopaminergic neurotoxicity. Glia, 55, 1362–1373.
Zhang, D., Hu, X., Qian, L., Chen, S. H., Zhou, H., Wilson, B., et al. (2011). Microglial MAC1 receptor and PI3K are essential in mediating beta-amyloid peptide-induced microglial activation and subsequent neurotoxicity. Journal of Neuroinflammation, 8, 3.
Magnus, T., Chan, A., Savill, J., Toyka, K. V., & Gold, R. (2002). Phagocytotic removal of apoptotic, inflammatory lymphocytes in the central nervous system by microglia and its functional implications. Journal of Neuroimmunology, 130, 1–9.
Smith, M. E. (1999). Phagocytosis of myelin in demyelinative disease: A review. Neurochemical Research, 1999(24), 261–268.
Kacimi, R., Giffard, R. G., & Yenari, M. A. (2011). Endotoxin-activated microglia injure brain derived endothelial cells via NF-kappaB, JAK-STAT and JNK stress kinase pathways. Journal of Inflammation (London), 8, 7.
Kohji, T., & Matsumoto, Y. (2000). Coexpression of Fas/FasL and Bax on brain and infiltrating T cells in the central nervous system is closely associated with apoptotic cell death during autoimmune encephalomyelitis. Journal of Neuroimmunology, 106, 165–171.
Aloisi, F. (2001). Immune function of microglia. Glia, 36, 165–179.
Carpentier, P. A., Duncan, D. S., & Miller, S. D. (2008). Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain, Behavior, and Immunity, 22, 140–147.
O’Brien, K., Fitzgerald, D. C., Naiken, K., Alugupalli, K. R., Rostami, A. M., & Gran, B. (2008). Role of the innate immune system in autoimmune inflammatory demyelination. Current Medicinal Chemistry, 15, 1105–1115.
Lehnardt, S., Massillon, L., Follett, P., Jensen, F. E., Ratan, R., Rosenberg, P. A., et al. (2003). Activation of innate immunity in the CNS triggers neurodegeneration through a toll-like receptor 4-dependent pathway. Proceedings of the National Academy of Sciences of the United States of America, 100, 8514–8519.
Aloisi, F., Ria, F., & Adorini, L. (2000). Regulation of T-cell responses by CNS antigen-presenting cells: Different roles for microglia and astrocytes. Immunology Today, 21, 141–147.
Dheen, S. T., Kaur, C., & Ling, E. A. (2007). Microglial activation and its implications in the brain diseases. Current Medicinal Chemistry, 14, 1189–1197.
Nakamura, Y. (2002). Regulating factors for microglial activation. Biological & Pharmaceutical Bulletin, 25, 945–953.
Jang, S., Kelley, K. W., & Johnson, R. W. (2008). Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proceedings of the National Academy of Sciences of the United States of America, 105, 7534–7539.
Lin, M. W., Tsao, L. T., Chang, L. C., Chen, Y. L., Huang, L. J., Kuo, S. C., et al. (2007). Inhibition of lipopolysaccharide-stimulated NO production by a novel synthetic compound CYL-4d in RAW 264.7 macrophages involving the blockade of MEK4/JNK/AP-1 pathway. Biochemical Pharmacology, 73, 1796–1806.
Waetzig, V., Czeloth, K., Hidding, U., Mielke, K., Kanzow, M., Brecht, S., et al. (2005). c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia, 50, 235–246.
Minogue, A. M., Barrett, J. P., & Lynch, M. A. (2012). LPS-induced release of IL-6 from glia modulates production of IL-1 beta in a JAK2-dependent manner. Journal of Neuroinflammation, 9, 126.
Qin, H., Roberts, K. L., Niyongere, S. A., Cong, Y., Elson, C. O., & Benveniste, E. N. (2007). Molecular mechanism of lipopolysaccharide-induced SOCS-3 gene expression in macrophages and microglia. The Journal of Immunology, 179, 5966–5976.
Cassatella, M. A., Gasperini, S., Bovolenta, C., Calzetti, F., Vollebregt, M., Scapini, P., et al. (1999). Interleukin-10 (IL-10) selectively enhances CIS3/SOCS3 mRNA expression in human neutrophils: Evidence for an IL-10-induced pathway that is independent of STAT protein activation. Blood, 94, 2880–2889.
Niemand, C., Nimmesgern, A., Haan, S., Fischer, P., Schaper, F., Rossaint, R., et al. (2003). Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. The Journal of Immunology, 170, 3263–3272.
Qin, H., Wilson, C. A., Roberts, K. L., Baker, B. J., Zhao, X., & Benveniste, E. N. (2006). IL-10 inhibits lipopolysaccharide-induced CD40 gene expression through induction of suppressor of cytokine signaling-3. The Journal of Immunology, 177, 7761–7771.
Wong, P. K., Egan, P. J., Croker, B. A., O’Donnell, K., Sims, N. A., Drake, S., et al. (2006). SOCS-3 negatively regulates innate and adaptive immune mechanisms in acute IL-1-dependent inflammatory arthritis. Journal of Clinical Investigation, 116, 1571–1581.
El Kasmi, K. C., Holst, J., Coffre, M., Mielke, L., de, P. A., Lhocine, N., et al. (2006). General nature of the STAT3-activated anti-inflammatory response. The Journal of Immunology, 177, 7880–7888.
Murray, P. J. (2005). The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proceedings of the National Academy of Sciences of the United States of America, 2005(102), 8686–8691.
Staples, K. J., Smallie, T., Williams, L. M., Foey, A., Burke, B., Foxwell, B. M., et al. (2007). IL-10 induces IL-10 in primary human monocyte-derived macrophages via the transcription factor Stat3. The Journal of Immunology, 178, 4779–4785.
Fabrizi, C., Pompili, E., Panetta, B., Nori, S. L., & Fumagalli, L. (2009). Protease-activated receptor-1 regulates cytokine production and induces the suppressor of cytokine signaling-3 in microglia. International Journal of Molecular Medicine, 24, 367–371.
Cohen, S. J., Cohen, I. R., & Nussbaum, G. (2010). IL-10 mediates resistance to adoptive transfer experimental autoimmune encephalomyelitis in MyD88(−/−) mice. The Journal of Immunology, 184, 212–221.
Mirshafiey, A., & Mohsenzadegan, M. (2009). TGF-beta as a promising option in the treatment of multiple sclerosis. Neuropharmacology, 56, 929–936.
Li, Y., Chu, N., Rostami, A., & Zhang, G. X. (2006). Dendritic cells transduced with SOCS-3 exhibit a tolerogenic/DC2 phenotype that directs type 2 Th cell differentiation in vitro and in vivo. The Journal of Immunology, 177, 1679–1688.
Nguyen, T. L., Sullivan, N. L., Ebel, M., Teague, R. M., & DiPaolo, R. J. (2011). Antigen-specific TGF-beta-induced regulatory T cells secrete chemokines, regulate T cell trafficking, and suppress ongoing autoimmunity. The Journal of Immunology, 187, 1745–1753.
Lucchinetti, C., Bruck, W., Parisi, J., Scheithauer, B., Rodriguez, M., & Lassmann, H. (2000). Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Annals of Neurology, 47, 707–717.
Patani, R., Balaratnam, M., Vora, A., & Reynolds, R. (2007). Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathology and Applied Neurobiology, 33, 277–287.
Patrikios, P., Stadelmann, C., Kutzelnigg, A., Rauschka, H., Schmidbauer, M., Laursen, H., et al. (2006). Remyelination is extensive in a subset of multiple sclerosis patients. Brain, 129, 3165–3172.
Choi, D. K., Koppula, S., & Suk, K. (2011). Inhibitors of microglial neurotoxicity: Focus on natural products. Molecules, 16, 1021–1043.
Aktas, O., Prozorovski, T., & Zipp, F. (2006). Death ligands and autoimmune demyelination. Neuroscientist, 12, 305–316.
Austin, J. W., & Fehlings, M. G. (2008). Molecular mechanisms of Fas-mediated cell death in oligodendrocytes. Journal of Neurotrauma, 25, 411–426.
Li, W., Maeda, Y., Ming, X., Cook, S., Chapin, J., Husar, W., et al. (2002). Apoptotic death following Fas activation in human oligodendrocyte hybrid cultures. Journal of Neuroscience Research, 69, 189–196.
Gallo, V., & Armstrong, R. C. (2008). Myelin repair strategies: A cellular view. Current Opinion in Neurology, 21, 278–283.
Acknowledgments
This work was supported by the China National Natural Science Fund Project No. 812272791.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, W., Shao, J., Lu, H. et al. Guard of Delinquency? A Role of Microglia in Inflammatory Neurodegenerative Diseases of the CNS. Cell Biochem Biophys 70, 1–8 (2014). https://doi.org/10.1007/s12013-014-9872-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-9872-0