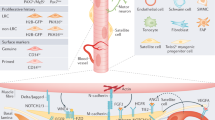
Abstract
The myogenic stem cell (satellite cell) is almost solely responsible for the remarkable regeneration of adult skeletal muscle fibers after injury. The availability and the functionality of satellite cells are the determinants of efficient muscle regeneration. During aging, the efficiency of muscle regeneration declines, suggesting that the functionality of satellite cells and their progeny may be altered. Satellite cells do not sit in isolation but rather are surrounded by, and influenced by, many extrinsic factors within the muscle tissue that can alter their functionality. These factors likely change during aging and impart both reversible and irreversible changes to the satellite cells and on their proliferating progeny. In this review, we discuss the possible mechanisms of impaired muscle regeneration with respect to the biology of satellite cells. Future studies that enhance our understanding of the interactions between stem cells and the environment in which they reside will offer promise for therapeutic applications in age-related diseases.

Similar content being viewed by others
References
Sadeh, M., Czyewski, K., & Stern, L. Z. (1985). Chronic myopathy induced by repeated bupivacaine injections. Journal of the Neurological Sciences, 67, 229–238.
Carlson, B. M., & Faulkner, J. A. (1989). Muscle transplantation between young and old rats: age of host determines recovery. American Journal Physiology, 256, C1262–C1266.
McGeachie, J. K., & Grounds, M. D. (1995). Retarded myogenic cell replication in regenerating skeletal muscles of old mice: An autoradiographic study in young and old BALBc and SJL/J mice. Cell & Tissue Research, 280, 277–282.
Carlson, B. M., Dedkov, E. I., Borisov, A. B., & Faulkner, J. A. (2001). Skeletal muscle regeneration in very old rats. J Gerontol Ser A Biol Sci Med Sci, 56, B224–B233.
Conboy, I. M., Conboy, M. J., Smythe, G. M., & Rando, T. A. (2003). Notch-mediated restoration of regenerative potential to aged muscle. Science, 302, 1575–1577.
Brack, A. S., Conboy, M. J., Roy, S., Lee, M., Kuo, C. J., Keller, C., et al. (2007). Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science, 317, 807–810.
Hawke, T. J., & Garry, D. J. (2001). Myogenic satellite cells: Physiology to molecular biology. Journal Applied Physiology, 91, 534–551.
Chargé, S. B., & Rudnicki, M. A. (2004). Cellular and molecular regulation of muscle regeneration. Physiological Reviews, 84, 209–238.
Zammit, P. S., Partridge, T. A., & Yablonka-Reuveni, Z. (2006). The skeletal muscle satellite cell: The stem cell that came in from the cold. Journal of Histochemistry and Cytochemistry, 54, 1177–1191.
Mauro, A. (1961). Satellite cells of skeletal muscle fibers. Journal of Biophysical and Biochemical Cytology, 9, 493–495.
Tamaki, T., Akatsuka, A., Ando, K., Nakamura, Y., Matsuzawa, H., Hotta, T., et al. (2002a). Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. Journal Cell Biology, 157, 571–577.
Tamaki, T., Akatsuka, A., Yoshimura, S., Roy, R. R., & Edgerton, V. R. (2002b). New fiber formation in the interstitial spaces of rat skeletal muscle during postnatal growth. Journal of Histochemistry and Cytochemistry, 50, 1097–1111.
De Angelis, L., Berghella, L., Coletta, M., Lattanzi, L., Zanchi, M., Cusella-De Angelis, M. G., et al. (1999). Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. Journal of Cell Biology, 147, 869–878.
McKinney-Freeman, S. L., Jackson, K. A., Camargo, F. D., Ferrari, G., Mavilio, F., & Goodell, M. A. (2002). Muscle-derived hematopoietic stem cells are hematopoietic in origin. Proceedings of the National Academy of Sciences of the United States of America, 99, 1341–1346.
Polesskaya, A., Seale, P., & Rudnicki, M. A. (2003). Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell, 113, 841–852.
Galvez, B. G., Sampaolesi, M., Brunelli, S., Covarello, D., Gavina, M., Rossi, B., et al. (2006). Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability. Journal of Cell Biology, 174, 231–243.
Zammit, P., & Beauchamp, J. (2001). The skeletal muscle satellite cell: Stem cell or son of stem cell? Differentiation, 68, 193–204.
Beauchamp, J. R., Heslop, L., Yu, D. S., Tajbakhsh, S., Kelly, R. G., Wernig, A., et al. (2000). Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. Journal Cell Biology, 151, 1221–1234.
Cornelison, D. D., Filla, M. S., Stanley, H. M., Rapraeger, A. C., & Olwin, B. B. (2001). Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Developments in Biologicals, 239, 79–94.
Seale, P., Ishibashi, J., Scime, A., & Rudnicki, M. A. (2004). Pax7 Is necessary and sufficient for the myogenic specification of CD45(+):Sca1(+) stem cells from injured muscle. PLoS Biol, 2, E130.
Fukada, S. I., Uezumi, A., Ikemoto, M., Masuda, S., Segawa, M., Tanimura, N., et al. (2007). Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells (in press).
Kuang, S., Charge, S. B., Seale, P., Huh, M., & Rudnicki, M. A. (2006). Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. Journal of Cell Biology, 172, 103–113.
Kuang, S., Kuroda, K., Le, G. F., & Rudnicki, M. A. (2007). Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell, 129, 999–1010.
Jackson, K. A., Mi, T., & Goodell, M. A. (1999). Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America, 96, 14482–14486.
Asakura, A., Seale, P., Girgis-Gabardo, A., & Rudnicki, M. A. (2002). Myogenic specification of side population cells in skeletal muscle. Journal of Cell Biology, 159, 123–134.
Uezumi, A., Ojima, K., Fukada, S., Ikemoto, M., Masuda, S., Miyagoe-Suzuki, Y., et al. (2006). Functional heterogeneity of side population cells in skeletal muscle. Biochemical and biophysical research communications, 341, 864–873.
Zammit, P. S., Heslop, L., Hudon, V., Rosenblatt, J. D., Tajbakhsh, S., Buckingham, M. E., et al. (2002). Kinetics of myoblast proliferation show that resident satellite cells are competent to fully regenerate skeletal muscle fibers. Experimental Cell Research, 281, 39–49.
Brack, A. S., Bildsoe, H., & Hughes, S. M. (2005). Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. Journal Cell Science, 118, 4813–4821.
Collins, C. A., Zammit, P. S., Ruiz, A. P., Morgan, J. E., & Partridge, T. A. (2007). A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells, 25, 885–894.
Schultz, E., Gibson, M. C., & Champion, T. (1978). Satellite cells are mitotically quiescent in mature mouse muscle: An EM and radioautographic study. Journal Experimental Zoology, 206, 451–456.
Bischoff, R. (1975). Regeneration of single skeletal muscle fibers in vitro. Anatomical Record, 182, 215–235.
Rosenblatt, J. D., Lunt, A. I., Parry, D. J., & Partridge, T. A. (1995). Culturing satellite cells from living single muscle fiber explants. In Vitro Cell Dev Biol, 31, 773–779.
Collins, C. A., Olsen, I., Zammit, P. S., Heslop, L., Petrie, A., Partridge, T. A., et al. (2005). Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell, 122, 289–301.
Cardasis, C. A., & Cooper, G. W. (1975). An analysis of nuclear numbers in individual muscle fibers during differentiation and growth: A satellite cell-muscle fiber growth unit. Journal Experimental Zoology, 191, 347–358.
Shefer, G., Van de Mark, D. P., Richardson, J. B., & Yablonka-Reuveni, Z. (2006). Satellite-cell pool size does matter: Defining the myogenic potency of aging skeletal muscle. Developments in Biologicals, 294, 50–66.
Schultz, E., & Lipton, B. H. (1982). Skeletal muscle satellite cells: Changes in proliferation potential as a function of age. Mechanism of Ageing and Development, 20, 377–383.
Bockhold, K. J., Rosenblatt, J. D., & Partridge, T. A. (1998). Aging normal and dystrophic mouse muscle: Analysis of myogenicity in cultures of living single fibers. Muscle Nerve, 21, 173–183.
Roth, S. M., Martel, G. F., Ivey, F. M., Lemmer, J. T., Metter, E. J., Hurley, B. F., et al. (2000). Skeletal muscle satellite cell populations in healthy young and older men and women. Anatomical Record, 260, 351–358.
Sajko, S., Kubinova, L., Cvetko, E., Kreft, M., Wernig, A., & Erzen, I. (2004). Frequency of M-cadherin-stained satellite cells declines in human muscles during aging. Journal of Histochemistry and Cytochemistry, 52, 179–185.
Gibson, M. C., & Schultz, E. (1983). Age-related differences in absolute numbers of skeletal muscle satellite cells. Muscle Nerve, 6, 574–580.
Snow, M. H. (1977). The effects of aging on satellite cells in skeletal muscles of mice and rats. Cell & Tissue Research, 185, 399–408.
Conboy, I. M., Conboy, M. J., Wagers, A. J., Girma, E. R., Weissman, I. L., & Rando, T. A. (2005). Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature, 433, 760–764.
Conboy, I. M., & Rando, T. A. (2005). Aging, stem cells and tissue regeneration: Lessons from muscle. Cell Cycle, 4, 407–410.
Bekaert, S., Derradji, H., & Baatout, S. (2004). Telomere biology in mammalian germ cells and during development. Developments in Biologicals, 274, 15–30.
Campisi, J. (2001). From cells to organisms: Can we learn about aging from cells in culture? Experimental Gerontology, 36, 607–618.
Decary, S., Mouly, V., Hamida, C. B., Sautet, A., Barbet, J. P., & Butler-Browne, G. S. (1997). Replicative potential and telomere length in human skeletal muscle: Implications for satellite cell-mediated gene therapy. Human Gene Therapy, 8, 1429–1438.
Decary, S., Hamida, C. B., Mouly, V., Barbet, J. P., Hentati, F., & Butler-Browne, G. S. (2000). Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscular Disorders, 10, 113–120.
Mouly, V., Aamiri, A., Bigot, A., Cooper, R. N., Di, D. S., Furling, D., et al. (2005). The mitotic clock in skeletal muscle regeneration, disease and cell mediated gene therapy. Acta Physiologica Scandinavica, 184, 3–15.
Bortoli, S., Renault, V., Eveno, E., Auffray, C., Butler-Browne, G., & Pietu, G. (2003). Gene expression profiling of human satellite cells during muscular aging using cDNA arrays. Gene, 321, 145–154.
Palacios, D., & Puri, P. L. (2006). The epigenetic network regulating muscle development and regeneration. Journal of Cellular Physiology, 207, 1–11.
Chambers, S. M., Shaw, C. A., Gatza, C., Fisk, C. J., Donehower, L. A., & Goodell, M. A. (2007). Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol, 5, e201.
Barani, A. E., Durieux, A. C., Sabido, O., & Freyssenet, D. (2003). Age-related changes in the mitotic and metabolic characteristics of muscle-derived cells. Journal of Applied Physiology, 95, 2089–2098.
Conboy, I. M., & Rando, T. A. (2002). The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Developments in Cell Biology, 3, 397–409.
Chargé, S. B., Brack, A. S., & Hughes, S. M. (2002). Aging-related satellite cell differentiation defect occurs prematurely after Ski-induced muscle hypertrophy. American Journal of Physiology Cell Physiol, 283, C1228–C1241.
Asakura, A., Komaki, M., & Rudnicki, M. (2001). Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation, 68, 245–253.
Wada, M. R., Inagawa-Ogashiwa, M., Shimizu, S., Yasumoto, S., & Hashimoto, N. (2002). Generation of different fates from multipotent muscle stem cells. Development, 129, 2987–2995.
Shefer, G., Wleklinski-Lee, M., & Yablonka-Reuveni, Z. (2004). Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. Journal of Cell Science, 117, 5393–5404.
Vertino, A. M., Taylor-Jones, J. M., Longo, K. A., Bearden, E. D., Lane, T. F., McGehee, R. E., Jr., et al. (2005). Wnt10b deficiency promotes coexpression of myogenic and adipogenic programs in myoblasts. Molecular Biology of the Cell, 16, 2039–2048.
Taylor-Jones, J. M., McGehee, R. E., Rando, T. A., Lecka-Czernik, B., Lipschitz, D. A., & Peterson, C. A. (2002). Activation of an adipogenic program in adult myoblasts with age. Mechanism of Ageing and Development, 123, 649–661.
Jejurikar, S. S., Henkelman, E. A., Cederna, P. S., Marcelo, C. L., Urbanchek, M. G., & Kuzon, W. M., Jr. (2006). Aging increases the susceptibility of skeletal muscle derived satellite cells to apoptosis. Experimental Gerontology, 41, 828–836.
Sidorenko, A. V., Gubrii, I. B., Andrianova, L. F., Macsijuk, T. V., & Butenko, G. M. (1986). Functional rearrangement of lymphohemopoietic system in heterochronically parabiosed mice. Mechanism of Ageing and Development, 36, 41–56.
Hirayama, R., Takemura, K., Nihei, Z., Ichikawa, W., Takagi, Y., Mishima, Y., et al. (1993). Differential effect of host microenvironment and systemic humoral factors on the implantation and the growth rate of metastatic tumor in parabiotic mice constructed between young and old mice. Mechanism of Ageing and Development, 71, 213–221.
Fujino, H., Kohzuki, H., Takeda, I., Kiyooka, T., Miyasaka, T., Mohri, S., et al. (2005). Regression of capillary network in atrophied soleus muscle induced by hindlimb unweighting. Journal Applied Physiology, 98, 1407–1413.
Ryan, N. A., Zwetsloot, K. A., Westerkamp, L. M., Hickner, R. C., Pofahl, W. E., & Gavin, T. P. (2006). Lower skeletal muscle capillarization and VEGF expression in aged vs. young men. Journal Applied Physiology, 100, 178–185.
Goldspink, G., Fernandes, K., Williams, P. E., & Wells, D. J. (1994). Age-related changes in collagen gene expression in the muscles of mdx dystrophic and normal mice. Neuromuscular Disorders, 4, 183–191.
Alexakis, C., Partridge, T., & Bou-Gharios, G. (2007). Implication of the satellite cell in dystrophic muscle fibrosis: A self perpetuating mechanism of collagen over-production. American Journal of Physiology Cell Physiology.
Engler, A. J., Sen, S., Sweeney, H. L., & Discher, D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell, 126, 677–689.
Bischoff, R. (1986). A satellite cell mitogen from crushed adult muscle. Developments in Biologicals, 115, 140–147.
Chen, G., & Quinn, L. S. (1992). Partial characterization of skeletal myoblast mitogens in mouse crushed muscle extract. Journal of Cellular Physiology, 153, 563–574.
Yablonka-Reuveni, Z., Seger, R., & Rivera, A. J. (1999). Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. Journal of Histochemistry and Cytochemistry, 47, 23–42.
Sheehan, S. M., Tatsumi, R., Temm-Grove, C. J., & Allen, R. E. (2000). HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve, 23, 239–245.
Quinn, L. S., Ong, L. D., & Roeder, R. A. (1990). Paracrine control of myoblast proliferation and differentiation by fibroblasts. Developments in Biologicals, 140, 8–19.
Pampusch, M. S., Hembree, J. R., Hathaway, M. R., & Dayton, W. R. (1990). Effect of transforming growth factor beta on proliferation of L6 and embryonic porcine myogenic cells. Journal of Cellular Physiology, 143, 524–528.
Vandenburgh, H. H., Sheff, M. F., & Zacks, S. I. (1984). Soluble age-related factors from skeletal muscle which influence muscle development. Experimental Cell Research, 153, 389–401.
Mezzogiorno, A., Coletta, M., Zani, B. M., Cossu, G., & Molinaro, M. (1993). Paracrine stimulation of senescent satellite cell proliferation by factors released by muscle or myotubes from young mice. Mechanism of Ageing and Development, 70, 35–44.
Beggs, M. L., Nagarajan, R., Taylor-Jones, J. M., Nolen, G., Macnicol, M., & Peterson, C. A. (2004). Alterations in the TGFbeta signaling pathway in myogenic progenitors with age. Aging Cell, 3, 353–361.
Li, Y., Foster, W., Deasy, B. M., Chan, Y., Prisk, V., Tang, Y., et al. (2004). Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: A key event in muscle fibrogenesis. American Journal of Pathology, 164, 1007–1019.
Florini, J. R., & Magri, K. A. (1989). Effects of growth factors on myogenic differentiation. American Journal Physiology, 256, C701–C711.
Reeves, I., Abribat, T., Laramee, P., Jasmin, G., & Brazeau, P. (2000). Age-related serum levels of insulin-like growth factor-I, -II and IGF-binding protein-3 following myocardial infarction. Growth Hormone & IGF Research, 10, 78–84.
Liu, H., Fergusson, M. M., Castilho, R. M., Liu, J., Cao, L., Chen, J., et al. (2007). Augmented Wnt signaling in a mammalian model of accelerated aging. Science, 317, 803–806.
Chen, Y., Zajac, J. D., & MacLean, H. E. (2005). Androgen regulation of satellite cell function. Journal Endocrinology, 186, 21–31.
Snyder, P. J. (2001). Effects of age on testicular function and consequences of testosterone treatment. Journal of clinical endocrinology and metabolism, 86, 2369–2372.
Renault, V., Rolland, E., Thornell, L. E., Mouly, V., & Butler-Browne, G. (2002). Distribution of satellite cells in the human vastus lateralis muscle during aging. Experimental Gerontology, 37, 1513–1514.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brack, A.S., Rando, T.A. Intrinsic Changes and Extrinsic Influences of Myogenic Stem Cell Function During Aging. Stem Cell Rev 3, 226–237 (2007). https://doi.org/10.1007/s12015-007-9000-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-007-9000-2