Abstract
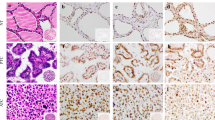
Long non-coding RNAs (lncRNAs) may contribute to carcinogenesis and tumor progression by regulating transcription and gene expression. The role of lncRNAs in the regulation of thyroid cancer progression is being extensively examined. Here, we analyzed three lncRNAs that were overexpressed in papillary thyroid carcinomas, long intergenic non-protein coding RNA, regulator of reprogramming (Linc-ROR, ROR) PVT1 oncogene (PVT1), and HOX transcript antisense intergenic RNA (HOTAIR) to determine their roles in thyroid tumor development and progression. ROR expression has not been previously examined in thyroid carcinomas. Tissue microarrays (TMAs) of formalin-fixed paraffin-embedded tissue sections from 129 thyroid cases of benign and malignant tissues were analyzed by in situ hybridization (ISH), automated image analysis, and real-time PCR. All three lncRNAs were most highly expressed in the nuclei of PTCs. SiRNA experiments with a PTC cell line, TPC1, showed inhibition of proliferation with siRNAs for all three lncRNAs while invasion was inhibited with siRNAs for ROR and HOTAIR. SiRNA experiments with ROR also led to increased expression of miR-145, supporting the role of ROR as an endogenous miR-145 sponge. After treatment with TGF-β, there was increased expression of ROR, PVT1, and HOTAIR in the PTC1 cell line compared to control groups, indicating an induction of their expression during epithelial to mesenchymal transition (EMT). These results indicate that ROR, PVT1, and HOTAIR have important regulatory roles during the development of PTCs.







Similar content being viewed by others
References
Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs, 4th Edition, International Agency for Research on Cancer (IARC) Lyon, France, 2017.
Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7(10):569–580.
Livoisi VA. Papillary thyroid carcinoma: An update. Mod Pathol. 2011;24(Suppl 2):S1–S9.
Li X, Wu Z, Fu X, Han W. Long noncoding RNAs: Insights from biological features and functions to diseases. Med Res Rev. 2013;33(3):517–553.
Zhang R, Hardin H, Chen J, Guo Z, Lloyd RV. Non-coding RNAs in Thyroid Cancer Endocr Pathol. 2016;27:12–20.
Sui F, Ji M, Hou P. Long non-coding RNAs in thyroid cancer: Biological functions and clinical significance. Molecular and Cellular Endocrin. 2017, https://doi.org/10.1016/j.mce.2017.07.020 (In Press).
He H, Nagy R, Liyanarachchi S, Jiao H, Li W, Suster S, Kere J, de la Chapelle A. A susceptibility locus for papillary thyroid carcinoma on chormosome 8q24. Cancer Res. 2009;69(2):625–631.
He H, Li W, Liyanarachchi S, Jendrzejewski J, Srinivas M, Davuluri RV, Nagy R, de la Chapelle A. Genetic predisposition to papillary thryoid carcinoma: Involvement of FOXE1, TSHR, and a Novel lincRNA Gene, PTCSC2. J Clin Endocrinol Metab. 2015;100(1):E164–E172.
Jendrzejewski J, He H, Randomska HS, Li W, Tomsic J, Liyanarachchi S, Davuluri RV, Nagy R, de la Chapelle A. The polymorphism rs944289 predisposes to papillary thryoid carcinoma through a large intergenic noncoding RNA gene of tumor suppressor type. Proc Natl Acad Sci USA. 2012;109(22):8646–8651.
Zhang R, Hardin H, Huang W, Chen J, Asioli S, Righi A, Maletta F, Sapino A, Lloyd RV. MALAT1 Long non-coding RNA expression in thyroid tissues: Analysis by in situ hybridizastion and real-time PCR. Endocr Pathology 2017;28:7–12.
Buehler D, Hardin H, Shan W, Montemayor-Garcia C, Rush PS, Asioli S, Chen H, Lloyd RV. Expression of epithelial-mesenchymal transition regulators SNAI2 and TWIST1 in thyroid carcinomas. Mod Pathol. 2012;26(10):54–61.
Guo Z, Hardin H, Montemayor-Garcia C, Asioli S, Righi A, Maletta F, Sapino A, Llloyd RV. In situ hybridization analysis of miR-146b-5p and miR-21 in thyroid nodules: Diagnostic implications. Endocr Pathol. 2015;26(2):157–163.
Huang W, Eickhoff JC, Mehraein-Ghomi F, Church DR, Wilding G, Basu HS. Expression of spermidine/spermine N1-acetyl transferase (SSAT) in human prostate tissues is related to prostate cancer progression and metastasis. Prostate. 2015;75(11):1150–1159.
Hardin H, Guo Z, Shan W, Montemayor-Garcia C, Asioli S, Yu XM, Harrison AD, Chen H, Lloyd RV. The roles of the epithelial-mesenchymal transition marker PRRX1 and miR-146b-5p in papillary thyroid carcinoma progression. Am J Pathol. 2014;184(8):2342–2354.
Hardin H, Yu X-M, Harrison AD, Larrain C, Zhang R, Chen J, Chen H, Lloyd RV. Generation of novel thyroid cancer stem-like cell clones effects of resveratrol and valproic acid. Am J Pathol. 2016;186(6):1662–1673.
Zhou Q, Chen J, Feng J, Wang J. Long non-coding RNA PVT1 modulates thyroid cancer cell proliferation by recruiting EZH2 and regulating thyroid-stimulation hormone receptor (TSHR). Tumor Biol 2016;37:3105–3113, https://doi.org/10.1007/s13277-015-4149-9
Di W, LiQ, Shen W, Guo H, Zhao S. The long non-coding RNA HOTAIR promotes thyroid cancer cell growth, invasion and migration through the miR-1-CCND2 axis. Am J Cancer Res. 2017;7:1298–1309.
Takahashi K, Yan IK, Haga H, Patel T. Modulation of hypoxia-signaling pathways by extracellular linc-ROR. J Cell Sci. 2014;127:1585–1594.
Zheng X, Hu H, Li S. High expression of lncRNA PVT1 promotes invasion by inducing epithelial-to-mesenchymal transition in esophageal cancer. Oncology Letters. 2016;12(4):2357–2362. https://doi.org/10.3892/ol.2016.5026.
Feng Xu, Jing Zhang, Long non-coding RNA HOTAIR functions as miRNA sponge to promote the epithelial to mesenchymal transition in esophageal cancer, Biomedicine & Pharmacotherapy, 90, 2017, 888–896, https://doi.org/10.1016/j.biopha.2017.03.103.
Pan Y, Li C, Chen J, Zhang K, Chu X, Wang R, Chen L. The emerging roles of long noncoding RNA ROR (lincRNA-ROR) and its possible mechanisms in human cancers. Cell Physiol Biochem. 2016;40:219–229.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipotsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715.
Hou P, Zhao Y, Li Z, Yao R, Ma M, Gao Y, Zhao L, Zhang Y, Huang B, Lu J. LincRNA-ROR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death and Disease. 2014;5: e1287; https://doi.org/10.1038/cddis.2014.249.
Cancer Genome Atlas Research Network. Integrated genomic characterizatin of papillary thyroid carcinoma. Cell. 2014;159(3):676–690.
Liu J, Singh B, Tallini G, Carlson DL, Katabi N, Shaha A, Tuttle RM, Ghossein RA. Follicular variant of papillary thyroid carcinoma: A clinicopathologtic study of a problematic entity. Cancer. 2006;107:1253–1264.
Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo FR, Thompson LD, Barletta JA, Wenig BM, Al Ghuzlan A, Kakudo K, Giordano TJ, Alves VA, Khanafshar E, Asa SL, El-Naggar AK, Gooding WE, Hodak SP, Lloyd RV, Maytal G, Mete O, Nikiforova MN, Nose V, Papotti M, Poller DN, Sadow PM, Tischler AS, Tuttle AS, Wall KB, LiVolsi VA, Randolph GW, Ghossein RA. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: A paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2:1023–1029.
Quiros RM, Ding HG, Gattuso P, Prinz RA, Xu X. Evidence that one subset of anaplastic thryoid carcinomas are derived from papillary carcinomas due to BRAF and p53 mutations. Cancer. 2005;103:2261–2268.
Hardin H, Montemayor-Garcia C, Lloyd RV. Thyroid cancer stem-like cells and epithelial-mesenchymal transition in thyroid cancers. Hum Pathol. 2013;44:1707–1713.
Zane M, Scavo E, Catalano V, Bonanno M, Todaro M, De Maria R, Stassi G. Normal vs cancer thyroid stem cells: The road to transformation. Oncogene. 2016;35:805–815.
Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thryoid cancers. J Clin Invest. 2016;126(3):1052–1066.
Braun J, Hoang-Vu C, Dralle H, Hutelmaier S. Downregulation of microRNAs directs the EMT and invasive potential of anapladstic thyroid carcinomas. Oncogene. 2010;29(29):4237–4244.
Visone R, Pallante P, Vecchione A, Cirombella R, Ferracin M, Ferraro A, Volinia S, Coluzzi S, Leone V, Borbone E, Liu CG, Petrocca F, Troncone G, Calin GA, Scarpa A, Colato C, Tallini G, Santoro M, Croce CM, Fusco A. Specific microRNAs are downregulated inhuman thyroid anaplastic carcinomas. Oncogene. 2007;26(54):7590–7595.
Maqsodi B, Nikoloff C. Non-isotopic method for in situ IncRNA visualization and quantitation. Methods Mol Biol. 2016;1402: 165–176.
Mehra R, Shi Y, Udager AM, Prensner JR, Sahu A, Iyer MK, Siddiqui J, Cao X, Wei J, Jiang H, Feng FY, Chinnaiyan AM. A novel RNA in situ hybridization assay for the long noncoding RNA SChLAP1 predicts poor clinical outcome after radical prostatectomy in clinical outcome after radical Prostatectomy in clinically localized prostate cancer. Neoplasia. 2014;16:1121–1127.
Acknowledgments
We kindly thank Dr. John A. Copland III (Mayo Clinic, Jacksonville, FL) for the THJ-16T and THJ-21T cell lines, Dr. Rebecca E. Schweppe (University of Colorado, Denver, CO) for the BCPAP cell line, Dr. Daniel T. Ruan (Brigham and Women’s Hospital, Boston, MA) for the TPC-1 cell line, the staff of the Translational Research in Pathology (TRIP), Flow Cytometry, 3P, and Experimental Pathology laboratories (University of Wisconsin Carbone Cancer Center Cancer Center Support Grant P30 CA014520).
Funding
This study was supported by a grant from the UW Carbone Cancer Center (RVL) and a resident research grant from the UW Pathology Department to RZ.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study received ethical approval from the local Institutional Review Board.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplemental Figure 1
ISH localization of HOTAIR and ROR expression in the thyroid TMA. The strongest labeling was in the PTC while the NT and ATC were similar. Bar = 100 μm. (PDF 820 kb)
Supplemental Figure 2
Three FFPE samples each from the NT, PTC and ATC groups were tested for the lncRNAs PTCSC1 (A) and SOX2OT (B) by qPCR. The PTC group was significantly downregulated for both lncRNAs compared to the NT group. Whereas, both lncRNAs were also downregulated to that of the ATC group, however, this finding was not significant. Error bars expressed as SEM. ** = p<0.01, ***=p<.001 (PDF 43 kb)
Supplemental Figure 3
TPC1 cells were transfected with 10, 30 and 50 nMol of siRNAs for PVT1, ROR and HOTAIR. Represented are optimal concentrations assessed by RT-qPCR. Error bars are expressed as SEM. * = p<0.05, ** = p<0.01. (PDF 43 kb)
Rights and permissions
About this article
Cite this article
Zhang, R., Hardin, H., Huang, W. et al. Long Non-coding RNA Linc-ROR Is Upregulated in Papillary Thyroid Carcinoma. Endocr Pathol 29, 1–8 (2018). https://doi.org/10.1007/s12022-017-9507-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-017-9507-2