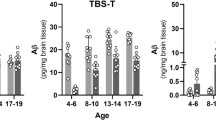
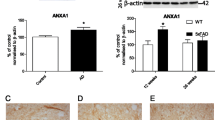
Abstract
Accumulation of beta-amyloid (Aβ) in the extracellular space, which is one of the hallmarks of Alzheimer’s disease (AD), depends on the balance between its synthesis and clearance. The physiological role of extracellular chaperones, capable of affecting early events in the amyloid cascade, is increasingly being investigated by many research groups. Among these proteins, we focused on haptoglobin, which we recently found to form a complex with beta-amyloid in brain tissues or cerebrospinal fluids from patients with AD. We also previously reported that haptoglobin increases with age in rat hippocampus. Major aim of this study was to evaluate whether haptoglobin influences Aβ interaction with astrocytes and its internalization into these cells. Haptoglobin effect on Aβ-induced cell death was also explored. We report here that haptoglobin impairs Aβ uptake by human glioblastoma–astrocytoma cell line U-87 MG and limits the toxicity of this peptide on these cells. Of note, our data also show that Aβ can stimulate haptoglobin release by astrocyte cell lines. The study of the risk of developing AD should be focused not only on the analysis of Aβ but also on the level of critical ligands, such as haptoglobin, able to influence peptide aggregation or clearance.






Similar content being viewed by others
References
Argüelles S, Venero JL, García-Rodriguez S, Ayala A, Cano J, Machado A (2010) Use of haptoglobin and transthyretin as potential biomarkers for the preclinical diagnosis of Parkinson’s disease. Neurochem Int 57:227–234
Basak JM, Verghese PB, Yoon H, Kim J, Holtzman DM (2012) Low-density lipoprotein receptor represents an apolipoprotein E-independent pathway of Aβ uptake and degradation by astrocytes. J Biol Chem 287:13959–13971
Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R et al (2007) Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab 27:909–918
Bertrand J, Begaud-Grimaud G, Bessette B, Verdier M, Battu S, Jauberteau MO (2009) Cancer stem cells from human glioma cell line are resistant to Fas-induced apoptosis. Int J Oncol 34:717–727
Bien-Ly N, Gillespie AK, Walker D, Yoon SY, Huang Y (2012) Reducing human apolipoprotein E levels attenuates age-dependent Aβ accumulation in mutant human amyloid precursor protein transgenic mice. J Neurosci 32:4803–4811
Bishop GM, Robinson SR (2002) The amyloid hypothesis: let sleeping dogmas lie? Neurobiol Aging 23:1101–1105
Bishop GM, Robinson SR (2004) Physiological roles of amyloid-beta and implications for its removal in Alzheimer’s disease. Drugs Aging 21:621–630
Borsody M, Burke A, Coplin W, Miller-Lotan R, Levy A (2006) Haptoglobin and the development of cerebral artery vasospasm after subarachnoid hemorrhage. Neurology 66:634–640
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 7:248–254
Brera B, Serrano A, de Ceballos ML (2000) beta-amyloid peptides are cytotoxic to astrocytes in culture: a role for oxidative stress. Neurobiol Dis 7:395–405
Butterfield DA (2002) Amyloid β-peptide(1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain: a review. Free Radic Res 36:1307–1313
Carrero I, Gonzalo MR, Martin B, Sanz-Anquela JM, Arévalo-Serrano J, Gonzalo-Ruiz A (2012) Oligomers of β-amyloid protein (Aβ1-42) induce the activation of cyclooxygenase-2 in astrocytes via an interaction with interleukin-1β, tumour necrosis factor-α, and a nuclear factor κ B mechanism in the rat brain. Exp Neurol 236:215–227
Castellano JM, Deane R, Gottesdiener AJ, Verghese PB, Stewart FR, West T et al (2012) Low-density lipoprotein receptor overexpression enhances the rate of brain-to-blood Aβ clearance in a mouse model of β-amyloidosis. Proc Natl Acad Sci U S A 109:15502–15507
Chamoun V, Zeman A, Blennow K, Fredman P, Wallin A, Keir G et al (2001) Haptoglobins as markers of blood-CSF barrier dysfunction: the findings in normal CSF. J Neurol Sci 182:117–121
Chen C-H, Albers JJ (1982) Characterization of proteoliposomes containing apoprotein a-I: a new substrate for the measurement of lecithin:cholesterol acyltransferase activity. J Lipid Res 23:680–691
Chen JH, Tsou TC, Chiu IM, Chou CC (2010) Proliferation inhibition, DNA damage, and cell-cycle arrest of human astrocytoma cells after acrylamide exposure. Chem Res Toxicol 23:1449–1458
Cigliano L, Spagnuolo MS, Abrescia P (2003) Quantitative variations of the isoforms in haptoglobin 1–2 and 2–2 individual phenotypes. Arch Biochem Biophys 416:227–237
Cigliano L, Pugliese CR, Spagnuolo MS, Palumbo R, Abrescia P (2009) Haptoglobin binds the antiatherogenic protein apolipoprotein E—impairment of apolipoprotein E stimulation of both lecithin:cholesterol acyltransferase activity and cholesterol uptake by hepatocytes. FEBS J 276:6158–6171
Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB et al (2008) apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest 118:4002–4013
Fuentealba RA, Liu Q, Zhang J, Kanekiyo T, Hu X, Lee JM et al (2010) Low-density lipoprotein receptor-related protein 1 (LRP1) mediates neuronal Abeta42 uptake and lysosomal trafficking. PLoS One 5:e11884
Funato H, Yoshimura M, Yamazaki T, Saido TC, Ito Y, Yokofujita J et al (1998) Astrocytes containing amyloid beta-protein (Abeta)-positive granules are associated with Abeta40-positive diffuse plaques in the aged human brain. Am J Pathol 152:983–992
Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De Bona P, Bruno V et al (2009) Beta-amyloid monomers are neuroprotective. J Neurosci 29:10582–10587
Giuffrida ML, Caraci F, De Bona P, Pappalardo G, Nicoletti F, Rizzarelli E et al (2010) The monomer state of beta-amyloid: where the Alzheimer’s disease protein meets physiology. Rev Neurosci 21:83–93
Guénette SY (2003) Astrocytes: a cellular player in Aβ clearance and degradation. Trends Mol Med 9:279–280
Holtzman DM, Morris JC, Goate AM (2011) Alzheimer’s disease: the challenge of the second century. Sci Transl Med 3:77
Huang HC, Jiang ZF (2009) Accumulated amyloid-beta peptide and hyperphosphorylated tau protein: relationship and links in Alzheimer’s disease. J Alzheimers Dis 16:15–27
Huang Y, Mucke L (2012) Alzheimer mechanisms and therapeutic strategies. Cell 148:1204–1222
Huang YC, Wu YR, Tseng MY, Chen YC, Hsieh SY, Chen CM (2011) Increased prothrombin, apolipoprotein A-IV, and haptoglobin in the cerebrospinal fluid of patients with Huntington’s disease. PLoS One 6:e15809
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. (2012) A Paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 4, 147ra111.
Janciauskiene S, Rubin H, Lukacs CM, Wright HT (1998) Alzheimer’s peptide Aβ1-42 binds to two β-sheets of α1-antichymotrypsin and transforms it from inhibitor to substrate. J Biol Chem 273:28360–28364
Johnson G, Brane D, Block W, van Kammen DP, Gurklis J, Peters JL et al (1992) Cerebrospinal fluid protein variations in common to Alzheimer’s disease and schizophrenia. Appl Theor Electrophor 3(2):47–53
Kanekiyo T, Liu CC, Shinohara M, Li J, Bu G (2012) LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer’s amyloid-beta. J Neurosci 32:16458–16465
Kim J, Jiang H, Park S, Eltorai AE, Stewart FR, Yoon H et al (2011) Haploinsufficiency of human APOE reduces amyloid deposition in a mouse model of amyloid-β amyloidosis. J Neurosci 31:18007–18012
Kontush A, Berndt C, Weber W, Akopyan V, Arlt S, Schippling S et al (2001) Amyloid-β is an antioxidant for lipoproteins in cerebrospinal fluid and plasma. Free Radic Biol Med 30:119–128
Lasagna-Reeves CA, Kayed R (2011) Astrocytes contain amyloid-β annular protofibrils in Alzheimer’s disease brains. FEBS Lett 585:3052–3057
Lee MY, Kim SY, Choi JS, Lee IH, Choi YS, Jin JY et al (2002) Upregulation of haptoglobin in reactive astrocytes after transient forebrain ischemia in rats. J Cereb Blood Flow Metab 22:1176–1180
Li Y, Cheng D, Cheng R, Zhu X, Wan T, Liu J et al (2014) Mechanisms of U87 Astrocytoma cell uptake and trafficking of monomeric versus protofibril Alzheimer’s disease amyloid-β proteins. PLoS One 9:e99939
Luo Y, Sunderland T, Wolozin B (1996) Physiologic levels of beta-amyloid activate phosphatidylinositol 3-kinase with the involvement of tyrosine phosphorylation. J Neurochem 67:978–987
Manelli AM, Bulfinch LC, Sullivan PM, LaDu MJ (2007) Abeta42 neurotoxicity in primary co-cultures: effect of apoE isoform and Abeta conformation. Neurobiol Aging 28:1139–1147
Mawuenyega KG, Sigurdson W, Ovod V, Morris JC, Yarasheski KE, Bateman RJ (2010) Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science 330:1774
Medeiros R, Laferla FM (2013) Astrocytes: conductors of the Alzheimer disease neuroinflammatory symphony. Exp Neurol 239:133–138
Moser JJ, Fritzler MJ (2010) The microRNA and messengerRNA profile of the RNA-induced silencing complex in human primary astrocyte and astrocytoma cells. PLoS One 5:e13445
Mulder SD, Nielsen HM, Blankenstein MA, Eikelenboom P, Veerhuis R (2014) Apolipoproteins E and J interfere with amyloid-beta uptake by primary human astrocytes and microglia in vitro. Glia 62:493–503
Nadal RC, Rigby SE, Viles JH (2008) Amyloid beta-Cu2+ complexes in both monomeric and fibrillar forms do not generate H2O2 catalytically but quench hydroxyl radicals. Biochemistry 47:11653–11664
Nagele RG, D’Andrea MR, Lee H, Venkataraman V, Wang HY (2003) Astrocytes accumulate A beta 42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res 971:197–209
Nielsen HM, Veerhuis R, Holmqvist B, Janciauskiene S (2009) Binding and uptake of A beta1-42 by primary human astrocytes in vitro. Glia 57:978–988
Nielsen HM, Mulder SD, Beliën JA, Musters RJ, Eikelenboom P, Veerhuis R (2010) Astrocytic A beta 1–42 uptake is determined by A beta-aggregation state and the presence of amyloid-associated proteins. Glia 58:1235–1246
Paresce DM, Ghosh RN, Maxfield FR (1996) Microglial cells internalize aggregates of the Alzheimer’s disease amyloid beta-protein via a scavenger receptor. Neuron 17:553–565
Pike CJ, Burdick D, Walencewicz AJ, Glabe CG, Cotman CW (1993) Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci 13:1676–1687
Powers JM, Schlaepfer WW, Willingham MC, Hall BJ (1981) An immunoperoxidase study of senile cerebral amyloidosis with pathogenetic considerations. J Neuropathol Exp Neurol 40:592–612
Puzzo D, Arancio O (2013) Amyloid-β peptide: Dr. Jekyll or Mr. Hyde? J Alzheimers Dis 33(Suppl 1):S111–S120
Quaye IK (2008) Haptoglobin, inflammation and disease. Trans R Soc Trop Med Hyg 102:735–742
Robinson SR, Bishop GM (2002) Aβ as a bioflocculant: Implications for the amyloid hypothesis of Alzheimer’s disease. Neurobiol Aging 23:1051–1072
Salvatore A, Cigliano L, Bucci EM, Corpillo D, Velasco S, Carlucci A et al (2007) Haptoglobin binding to apolipoprotein a-I prevents damage from hydroxyl radicals on its stimulatory activity of the enzyme lecithin-cholesterol acyl-transferase. Biochemistry 46:11158–11168
Salvatore A, Cigliano L, Carlucci A, Bucci EM, Abrescia P (2009) Haptoglobin binds apolipoprotein E and influences cholesterol esterification in the cerebrospinal fluid. J Neurochem 110:255–263
Spagnuolo MS, Maresca B, Mollica MP, Cavaliere G, Cefaliello C, Trinchese G, et al (2014a) Haptoglobin increases with age in rat hippocampus and modulates apolipoprotein E. Front Cell Neurosci 8, 212. doi:10.3389/fncel.2014.00212
Spagnuolo MS, Maresca B, La Marca V, Carrizzo A, Veronesi C, Cupidi C et al (2014b) Haptoglobin interacts with apolipoprotein e and Beta-amyloid and influences their crosstalk. ACS Chem Neurosci 5:837–847
Stine WB Jr, Dahlgren KN, Krafft GA, LaDu MJ (2003) In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis. J Biol Chem 278:11612–11622
Thal DR (2012) The role of astrocytes in amyloid β-protein toxicity and clearance. Exp Neurol 236:1–5
Thal DR, Schultz C, Dehghani F, Yamaguchi H, Braak H, Braak E (2000) Amyloid beta-protein (Abeta)-containing astrocytes are located preferentially near N-terminal-truncated Abeta deposits in the human entorhinal cortex. Acta Neuropathol 100:608–617
Vance JE, Hayashi H (2010) Formation and function of apolipoprotein E containing lipoproteins in the nervous system. Biochim Biophys Acta 1801:806–818
Veerhuis R, Van Breemen MJ, Hoozemans JM, Morbin M, Ouladhadj J, Tagliavini F et al (2003) Amyloid beta plaque-associated proteins C1q and SAP enhance the Abeta1-42 peptide-induced cytokine secretion by adult human microglia in vitro. Acta Neuropathol 105:135–144
Verbeek MM, de Waal RM, Schipper JJ, Van Nostrand WE (1997) Rapid degeneration of cultured human brain pericytes by amyloid beta protein. J Neurochem 68:1135–1141
Verghese PB, Castellano JM, Garai K, Wang Y, Jiang H, Shah A et al (2013) ApoE influences amyloid-β (Aβ) clearance despite minimal apoE/Aβ association in physiological conditions. Proc Natl Acad Sci U S A 110:e1807–e1816
White AR, Zheng H, Galatis D, Maher F, Hesse L, Multhaup G et al (1998) Survival of cultured neurons from amyloid precursor protein knock-out mice against Alzheimer’s amyloid-beta toxicity and oxidative stress. J Neurosci 18:6207–6217
Wilhelmus MM, Otte-Höller I, van Triel JJ, Veerhuis R, Maat-Schieman ML, Bu G et al (2007) Lipoprotein receptor-related protein-1 mediates amyloid-beta-mediated cell death of cerebrovascular cells. Am J Pathol 171:1989–1999
Williams TL, Serpell LC (2011) Membrane and surface interactions of Alzheimer’s Aβ peptide—insights into the mechanism of cytotoxicity. FEBS J 278:3905–3917
Wilson MR, Yerbury JJ, Poon S (2008) Potential roles of abundant extracellular chaperones in the control of amyloid formation and toxicity. Mol Biosyst 4:42–52
Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F et al (2003) Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med 9:453–457
Yankner BA, Duffy LK, Kirschner DA (1990) Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by Tachykinin neuropeptides. Science 250:279–282
Yerbury JJ, Wilson MR (2010) Extracellular chaperones modulate the effects of Alzheimer’s patient cerebrospinal fluid on Abeta(1–42) toxicity and uptake. Cell Stress Chaperones 15:115–121
Yerbury JJ, Rybchyn MS, Easterbrook-Smith SB, Henriques C, Wilson MR (2005) The acute phase protein haptoglobin is a mammalian extracellular chaperone with an action similar to clusterin. Biochemistry 44:10914–10925
Yerbury JJ, Kumita JR, Meehan S, Dobson CM, Wilson MR (2009) alpha2-Macroglobulin and haptoglobin suppress amyloid formation by interacting with prefibrillar protein species. J Biol Chem 284:4246–4254
Zhao X, Song S, Sun G, Strong R, Zhang J, Grotta JC et al (2009) Neuroprotective role of haptoglobin after intracerebral hemorrhage. J Neurosci 29:15819–15827
Acknowledgments
This research was supported by a grant from the Compagnia di San Paolo (Neuroscience Program; 3868 SD/SD-2008.2487). The authors have no conflict of interests to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maresca, B., Spagnuolo, M.S. & Cigliano, L. Haptoglobin Modulates Beta-Amyloid Uptake by U-87 MG Astrocyte Cell Line. J Mol Neurosci 56, 35–47 (2015). https://doi.org/10.1007/s12031-014-0465-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-014-0465-6