Abstract
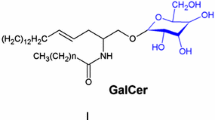
3-O-sulfogalactosylceramide or sulfatide is a major component of the myelin sheath in the central and peripheral nervous system. The examination of mice deficient in the sulfatide-synthesizing enzyme, cerebroside sulfotransferase, provided new insight into the role of sulfatide in the differentiation of myelinating cells, formation of the paranodal junction, and myelin maintenance. Although in general regarded as a marker for oligodendrocytes and Schwann cells, sulfatide is also present in astrocytes and neurons. The relatively low amount of sulfatide in neurons can dramatically increase in the absence of the sulfatide-degrading enzyme, arylsulfatase A, as in metachromatic leukodystrophy. Recent advance in the understanding of this disease comes from studies on new transgenic mouse models. Significant changes in sulfatide levels have also been observed more recently in Alzheimer’s disease and other diseases, suggesting that sulfatide might be involved in the pathogenesis of these diseases as well. This review summarizes recent studies on the physiological and pathophysiological role of sulfatide using transgenic mice deficient in its synthesis or degradation.

Similar content being viewed by others
References
Degroote S, Wolthoorn J, van Meer G (2004) The cell biology of glycosphingolipids. Semin Cell Dev Biol 15:375–387
Lahiri S, Futerman AH (2007) The metabolism and function of sphingolipids and glycosphingolipids. Cell Mol Life Sci 64:2270–2284
Hoetzl S, Sprong H, van Meer G (2007) The way we view cellular (glyco)sphingolipids. J Neurochem 103(Suppl 1):3–13
Thudichum JLW (1884) A treatise on the chemical constitution of the brain. Ballière, Tindall, and Cox, London
Vos JP, Lopes-Cardozo M, Gadella BM (1994) Metabolic and functional aspects of sulfogalactolipids. Biochim Biophys Acta 1211:125–149
Ishizuka I (1997) Chemistry and functional distribution of sulfoglycolipids. Prog Lipid Res 36:245–319
Senn C, Kutsche M, Saghatelyan A, Bösl MR, Löhler J, Bartsch U, Morellini F, Schachner M (2002) Mice deficient for the HNK-1 sulfotransferase show alterations in synaptic efficacy and spatial learning and memory. Mol Cell Neurosci 20:712–729
Gurevicius K, Gureviciene I, Sivukhina E, Irintchev A, Schachner M, Tanila H (2007) Increased hippocampal and cortical beta oscillations in mice deficient for the HNK-1 sulfotransferase. Mol Cell Neurosci 34:189–198
Norton WT, Cammer W (1984) Isolation and characterization of myelin. In: Morell P (ed) Myelin. Plenum, New York, NY, pp 147–195
Taylor CM, Marta CB, Bansal R, Pfeiffer SE (2004) The transport, assembly, and function of myelin lipids. In: Lazzarini RA (ed) Myelin biology and disorders. vol. 1. Academic, New York, NY, pp 57–88
Riebeling C, Allegood JC, Wang E, Merrill AH Jr, Futerman AH (2003) Two mammalian longevity assurance gene (LAG1) family members, trh1 and trh4, regulate dihydroceramide synthesis using different fatty acyl-CoA donors. J Biol Chem 278:43452–43459
Mizutani Y, Kihara A, Igarashi Y (2005) Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem J 390:263–271
Becker I, Wang-Eckhardt L, Yaghootfam A, Gieselmann V, Eckhardt M (2008) Differential expression of (dihydro)ceramide synthases in mouse brain: oligodendrocyte-specific expression of CerS2/Lass2. Histochem Cell Biol 129:233–241
Alderson NL, Maldonado EN, Kern MJ, Bhat NR, Hama H (2006) FA2H-dependent fatty acid 2-hydroxylation in postnatal mouse brain. J Lipid Res 47:2772–2780
Eckhardt M, Yaghootfam A, Fewou SN, Zöller I, Gieselmann V (2005) A mammalian fatty acid hydroxylase responsible for the formation of alpha-hydroxylated galactosylceramide in myelin. Biochem J 388:245–254
Alderson NL, Rembiesa BM, Walla MD, Bielawska A, Bielawski J, Hama H (2004) The human FA2H gene encodes a fatty acid 2-hydroxylase. J Biol Chem 279:48562–48568
Morell P, Radin NS (1969) Synthesis of cerebroside by brain from uridine diphosphate galactose and ceramide containing hydroxy fatty acid. Biochemistry 8:506–512
Basu S, Schultz AM, Basu M, Roseman S (1971) Enzymatic synthesis of galactocerebroside by a galactosyltransferase from embryonic chicken brain. J Biol Chem 246:4272–4279
van der Bijl P, Strous GJ, Lopes-Cardozo M, Thomas-Oates J, van Meer G (1996) Synthesis of non-hydroxy-galactosylceramides and galactosyldiglycerides by hydroxy-ceramide galactosyltransferase. Biochem J 317:589–597
Sprong H, Degroote S, Nilsson T, Kawakita M, Ishida N, van der Sluijs P, van Meer G (2003) Association of the Golgi UDP-galactose transporter with UDP-galactose:ceramide galactosyltransferase allows UDP-galactose import in the endoplasmic reticulum. Mol Biol Cell 14:3482–3493
Honke K, Tsuda M, Hirahara Y, Ishii A, Makita A, Wada Y (1997) Molecular cloning and expression of cDNA encoding human 3′-phosphoadenylylsulfate:galactosylceramide 3′-sulfotransferase. J Biol Chem 272:4864–4868
Hirahara Y, Tsuda M, Wada Y, Honke K (2000) cDNA cloning, genomic cloning, and tissue-specific regulation of mouse cerebroside sulfotransferase. Eur J Biochem 267:1909–1917
Yaghootfam A, Sorkalla T, Häberlein H, Gieselmann V, Kappler J, Eckhardt M (2007) Cerebroside sulfotransferase forms homodimers in living cells. Biochemistry 46:9260–9269
Kolter T, Sandhoff K (2005) Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu Rev Cell Dev Biol 21:81–103
Tempesta MC, Salvayre R, Levade T (1994) Functional compartments of sulphatide metabolism in cultured living cells: evidence for the involvement of a novel sulphatide-degrading pathway. Biochem J 297:479–489
Sundaram SK, Fan JH, Lev M (1995) A neutral galactocerebroside sulfate sulfatidase from mouse brain. J Biol Chem 270:10187–10192
Zeng Y, Cheng H, Jiang X, Han X (2008) Endosomes and lysosomes play distinct roles in sulfatide-induced neuroblastoma apoptosis: potential mechanisms contributing to abnormal sulfatide metabolism in related neuronal diseases. Biochem J 410:81–92
Berntson Z, Hansson E, Rönnbäck L, Fredman P (1998) Intracellular sulfatide expression in a subpopulation of astrocytes in primary cultures. J Neurosci Res 52:559–568
Pernber Z, Molander-Melin M, Berthold CH, Hansson E, Fredman P (2002) Expression of the myelin and oligodendrocyte progenitor marker sulfatide in neurons and astrocytes of adult rat brain. J Neurosci Res 69:86–93
Han X, Cheng H, Fryer JD, Fagan AM, Holtzman DM (2003) Novel role for apolipoprotein E in the central nervous system. Modulation of sulfatide content. J Biol Chem 278:8043–8051
Isaac G, Pernber Z, Gieselmann V, Hansson E, Bergquist J, Månsson JE (2006) Sulfatide with short fatty acid dominates in astrocytes and neurons. FEBS J 273:1782–1790
Schaeren-Wiemers N, van der Bijl P, Schwab ME (1995) The UDP-galactose:ceramide galactosyltransferase: expression pattern in oligodendrocytes and Schwann cells during myelination and substrate preference for hydroxyceramide. J Neurochem 65:2267–2278
Eckhardt M, Khalaj Hedayati K, Pitsch J, Lüllmann-Rauch R, Beck H, Fewou SN, Gieselmann V (2007) Sulfatide storage in neurons causes hyperexcitability and axonal degeneration in a mouse model of metachromatic leukodystrophy. J Neurosci 27:9009–9021
Han X (2007) Potential mechanisms contributing to sulfatide depletion at the earliest clinically recognizable stage of Alzheimer’s disease: a tale of shotgun lipidomics. J Neurochem 103(Suppl 1):171–179
Han X (2004) The role of apolipoprotein E in lipid metabolism in the central nervous system. Cell Mol Life Sci 61:1896–1906
Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW (1987) Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta 917:148–161
Boyles JK, Zoellner CD, Anderson LJ, Kosik LM, Pitas RE, Weisgraber KH, Hui DY, Mahley RW, Gebicke-Haerter PJ, Ignatius MJ, Shooter EM (1989) A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J Clin Invest 83:1015–1031
Vance JE, Karten B, Hayashi H (2006) Lipid dynamics in neurons. Biochem Soc Trans 34:399–403
Pfeiffer SE, Warrington AE, Bansal R (1993) The oligodendrocyte and its many cellular processes. Trends Cell Biol 3:191–197
Bansal R, Gard AL, Pfeiffer SE (1988) Stimulation of oligodendrocyte differentiation in culture by growth in the presence of a monoclonal antibody to sulfated glycolipid. J Neurosci Res 21:260–267
Bansal R, Pfeiffer SE (1989) Reversible inhibition of oligodendrocyte progenitor differentiation by a monoclonal antibody against surface galactolipids. Proc Natl Acad Sci U S A 86:6181–6185
Bansal R, Winkler S, Bheddah S (1999) Negative regulation of oligodendrocyte differentiation by galactosphingolipids. J Neurosci 19:7913–7924
Hirahara Y, Bansal R, Honke K, Ikenaka K, Wada Y (2004) Sulfatide is a negative regulator of oligodendrocyte differentiation: development in sulfatide-null mice. Glia 45:269–277
Pesheva P, Gloor S, Schachner M, Probstmeier R (1997) Tenascin-R is an intrinsic autocrine factor for oligodendrocyte differentiation and promotes cell adhesion by a sulfatide-mediated mechanism. J Neurosci 17:4642–4651
Roberts DD, Rao CN, Magnani JL, Spitalnik SL, Liotta LA, Ginsburg V (1985) Laminin binds specifically to sulfated glycolipids. Proc Natl Acad Sci U S A 82:1306–1310
Li S, Liquari P, McKee KK, Harrison D, Patel R, Lee S, Yurchenco PD (2005) Laminin-sulfatide binding initiates basement membrane assembly and enables receptor signaling in Schwann cells and fibroblasts. J Cell Biol 169:179–189
Chen LM, Bailey D, Fernandez-Valle C (2000) Association of beta 1 integrin with focal adhesion kinase and paxillin in differentiating Schwann cells. J Neurosci 20:3776–3784
Baron W, Decker L, Colognato H, Ffrench-Constant C (2003) Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr Biol 13:151–155
Coetzee T, Fujita N, Dupree J, Shi R, Blight A, Suzuki K, Suzuki K, Popko B (1996) Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell 86:209–219
Bosio A, Binczek E, Stoffel W (1996) Functional breakdown of the lipid bilayer of the myelin membrane in central and peripheral nervous system by disrupted galactocerebroside synthesis. Proc Natl Acad Sci U S A 93:13280–13285
Bosio A, Binczek E, Haupt WF, Stoffel W (1998) Composition and biophysical properties of myelin lipid define the neurological defects in galactocerebroside- and sulfatide-deficient mice. J Neurochem 70:308–315
Honke K, Hirahara Y, Dupree J, Suzuki K, Popko B, Fukushima K, Fukushima J, Nagasawa T, Yoshida N, Wada Y, Taniguchi N (2002) Paranodal junction formation and spermatogenesis require sulfoglycolipids. Proc Natl Acad Sci U S A 99:4227–4232
Marcus J, Honigbaum S, Shroff S, Honke K, Rosenbluth J, Dupree JL (2006) Sulfatide is essential for the maintenance of CNS myelin and axon structure. Glia 53:372–381
Ishibashi T, Dupree JL, Ikenaka K, Hirahara Y, Honke K, Peles E, Popko B, Suzuki K, Nishino H, Baba H (2002) A myelin galactolipid, sulfatide, is essential for maintenance of ion channels on myelinated axon but not essential for initial cluster formation. J Neurosci 22:6507–6514
Suzuki A, Hoshi T, Ishibashi T, Hayashi A, Yamaguchi Y, Baba H (2004) Paranodal axoglial junction is required for the maintenance of the Nav1.6-type sodium channel in the node of Ranvier in the optic nerves but not in peripheral nerve fibers in the sulfatide-deficient mice. Glia 46:274–283
Dupree JL, Mason JL, Marcus JR, Stull M, Levinson R, Matsushima GK, Popko B (2005) Oligodendrocytes assist in the maintenance of sodium channel clusters independent of the myelin sheath. Neuron Glia Biol 1:1–14
Hoshi T, Suzuki A, Hayashi S, Tohyama K, Hayashi A, Yamaguchi Y, Takeuchi K, Baba H (2007) Nodal protrusions, increased Schmidt–Lanterman incisures, and paranodal disorganization are characteristic features of sulfatide-deficient peripheral nerves. Glia 55:584–594
Hayashi A, Nakashima K, Yamagishi K, Hoshi T, Suzuki A, Baba H (2007) Localization of annexin II in the paranodal regions and Schmidt–Lanterman incisures in the peripheral nervous system. Glia 55:1044–1052
Simons M, Krämer EM, Thiele C, Stoffel W, Trotter J (2000) Assembly of myelin by association of proteolipid protein with cholesterol- and galactosylceramide-rich membrane domains. J Cell Biol 151:143–154
Schafer DP, Rasband MN (2006) Glial regulation of the axonal membrane at nodes of Ranvier. Curr Opin Neurobiol 16:508–514
Schafer DP, Bansal R, Hedstrom KL, Pfeiffer SE, Rasband MN (2004) Does paranode formation and maintenance require partitioning of neurofascin 155 into lipid rafts? J Neurosci 24:3176–3185
Schaeren-Wiemers N, Bonnet A, Erb M, Erne B, Bartsch U, Kern F, Mantei N, Sherman D, Suter U (2004) The raft-associated protein MAL is required for maintenance of proper axon-glia interactions in the central nervous system. J Cell Biol 166:731–742
Erne B, Sansano S, Frank M, Schaeren-Wiemers N (2002) Rafts in adult peripheral nerve myelin contain major structural myelin proteins and myelin and lymphocyte protein (MAL) and CD59 as specific markers. J Neurochem 82:550–562
Frank M, van der Haar ME, Schaeren-Wiemers N, Schwab ME (1998) rMAL is a glycosphingolipid-associated protein of myelin and apical membranes of epithelial cells in kidney and stomach. J Neurosci 18:4901–4913
Saravanan K, Schaeren-Wiemers N, Klein D, Sandhoff R, Schwarz A, Yaghootfam A, Gieselmann V, Franken S (2004) Specific downregulation and mistargeting of the lipid raft-associated protein MAL in a glycolipid storage disorder. Neurobiol Dis 16:396–406
Von Figura K, Gieselmann V, Jaeken J (2001) Metachromatic leukodystrophy. In: Scriver CR, Beaudet AL, Valle D, Sly WS (eds) The metabolic and molecular basis of inherited disease. McGraw Hill, New York, NY, pp 3695–372
Hess B, Saftig P, Hartmann D, Coenen R, Lüllmann-Rauch R, Goebel HH, Evers M, von Figura K, D’Hooge R, Nagels G, De Deyn P, Peters C, Gieselmann V (1996) Phenotype of arylsulfatase A-deficient mice: relationship to human metachromatic leukodystrophy. Proc Natl Acad Sci U S A 93:14821–14826
Gieselmann V, Matzner U, Hess B, Lüllmann-Rauch R, Coenen R, Hartmann D, D’Hooge R, DeDeyn P, Nagels G (1998) Metachromatic leukodystrophy: molecular genetics and an animal model. J Inherit Metab Dis 21:564–574
Wittke D, Hartmann D, Gieselmann V, Lüllmann-Rauch R (2004) Lysosomal sulfatide storage in the brain of arylsulfatase A-deficient mice: cellular alterations and topographic distribution. Acta Neuropathol 108:261–271
D’Hooge R, Hartmann D, Manil J, Colin F, Gieselmann V, De Deyn PP (1999) Neuromotor alterations and cerebellar deficits in aged arylsulfatase A-deficient transgenic mice. Neurosci Lett 273:93–96
D’Hooge R, Van Dam D, Franck F, Gieselmann V, De Deyn PP (2001) Hyperactivity, neuromotor defects, and impaired learning and memory in a mouse model for metachromatic leukodystrophy. Brain Res 907:35–43
Stroobants S, Leroy T, Eckhardt M, Aerts J-M, Berckmans D, D’Hooge R (2008) Early signs of neurolipidosis-related behavioural alterations in a murine model of metachromatic leukodystrophy. Behav Brain Res 189:306–316
Yaghootfam A, Gieselmann V, Eckhardt M (2005) Delay of myelin formation in arylsulphatase A-deficient mice. Eur J Neurosci 21:711–720
Consiglio A, Quattrini A, Martino S, Bensadoun JC, Dolcetta D, Trojani A, Benaglia G, Marchesini S, Cestari V, Oliverio A, Bordignon C, Naldini L (2001) In vivo gene therapy of metachromatic leukodystrophy by lentiviral vectors: correction of neuropathology and protection against learning impairments in affected mice. Nat Med 7:310–316
Sevin C, Benraiss A, Van Dam D, Bonnin D, Nagels G, Verot L, Laurendeau I, Vidaud M, Gieselmann V, Vanier M, De Deyn PP, Aubourg P, Cartier N (2006) Intracerebral adeno-associated virus-mediated gene transfer in rapidly progressive forms of metachromatic leukodystrophy. Hum Mol Genet 15:53–64
Sevin C, Verot L, Benraiss A, Van Dam D, Bonnin D, Nagels G, Fouquet F, Gieselmann V, Vanier MT, De Deyn PP, Aubourg P, Cartier N (2007) Partial cure of established disease in an animal model of metachromatic leukodystrophy after intracerebral adeno-associated virus-mediated gene transfer. Gene Ther 14:405–414
Matzner U, Harzer K, Learish RD, Barranger JA, Gieselmann V (2000) Long-term expression and transfer of arylsulfatase A into brain of arylsulfatase A-deficient mice transplanted with bone marrow expressing the arylsulfatase A cDNA from a retroviral vector. Gene Ther 7:1250–1257
Matzner U, Hartmann D, Lüllmann-Rauch R, Coenen R, Rothert F, Månsson JE, Fredman P, D’Hooge R, De Deyn PP, Gieselmann V (2002) Bone marrow stem cell-based gene transfer in a mouse model for metachromatic leukodystrophy: effects on visceral and nervous system disease manifestations. Gene Ther 9:53–63
Biffi A, De Palma M, Quattrini A, Del Carro U, Amadio S, Visigalli I, Sessa M, Fasano S, Brambilla R, Marchesini S, Bordignon C, Naldini L (2004) Correction of metachromatic leukodystrophy in the mouse model by transplantation of genetically modified hematopoietic stem cells. J Clin Invest 113:1118–1129
Biffi A, Capotondo A, Fasano S, del Carro U, Marchesini S, Azuma H, Malaguti MC, Amadio S, Brambilla R, Grompe M, Bordignon C, Quattrini A, Naldini L (2006) Gene therapy of metachromatic leukodystrophy reverses neurological damage and deficits in mice. J Clin Invest 116:3070–3082
Matzner U, Herbst E, Khalaj Hedayati K, Lüllmann-Rauch R, Wessig C, Schröder S, Eistrup C, Möller C, Fogh J, Gieselmann V (2005) Enzyme replacement improves nervous system pathology and function in a mouse model for metachromatic leukodystrophy. Hum Mol Genet 14:1139–1152
Givogri MI, Galbiati F, Fasano S, Amadio S, Perani L, Superchi D, Morana P, Del Carro U, Marchesini S, Brambilla R, Wrabetz L, Bongarzone E (2006) Oligodendroglial progenitor cell therapy limits central neurological deficits in mice with metachromatic leukodystrophy. J Neurosci 26:3109–3119
Klein D, Schmandt T, Muth-Köhne E, Perez-Bouza A, Segschneider M, Gieselmann V, Brüstle O (2006) Embryonic stem cell-based reduction of central nervous system sulfatide storage in an animal model of metachromatic leukodystrophy. Gene Ther 13:1686–1695
Gieselmann V, Matzner U, Klein D, Mansson JE, D’Hooge R, De Deyn PP, Lüllmann-Rauch R, Hartmann D, Harzer K (2003) Gene therapy: prospects for glycolipid storage diseases. Philos Trans R Soc Lond B Biol Sci 358:921–925
Matzner U, Gieselmann V (2005) Gene therapy of metachromatic leukodystrophy. Expert Opin Biol Ther 5:55–65
Sevin C, Aubourg P, Cartier N (2007) Enzyme, cell and gene-based therapies for metachromatic leukodystrophy. J Inherit Metab Dis 30:175–183
Biffi A, Naldini L (2007) Novel candidate disease for gene therapy: metachromatic leukodystrophy. Expert Opin Biol Ther 7:1193–1205
Lütjohann D, Harzer K, Gieselmann V, Eckhardt M (2006) Reduced brain cholesterol content in arylsulfatase A-deficient mice. Biochem Biophys Res Commun 344:647–650
Harzer K, Kustermann-Kühn B (1987) Brain galactolipid content in a patient with pseudoarylsulfatase A deficiency and coincidental diffuse disseminated sclerosis, and in patients with metachromatic, adreno-, and other leukodystrophies. J Neurochem 48:62–66
Poduslo SE, Miller K, Jang Y (1982) Biochemical studies of the late infantile form of metachromatic leukodystrophy. Acta Neuropathol 57:13–22
Walkley SU (2004) Secondary accumulation of gangliosides in lysosomal storage disorders. Semin Cell Dev Biol 15:433–444
Saher G, Brügger B, Lappe-Siefke C, Möbius W, Tozawa R, Wehr MC, Wieland F, Ishibashi S, Nave KA (2005) High cholesterol level is essential for myelin membrane growth. Nat Neurosci 8:468–475
Molander-Melin M, Pernber Z, Franken S, Gieselmann V, Månsson JE, Fredman P (2004) Accumulation of sulfatide in neuronal and glial cells of arylsulfatase A deficient mice. J Neurocytol 33:417–427
Schaeren-Wiemers N, Valenzuela DM, Frank M, Schwab ME (1995) Characterization of a rat gene, rMAL, encoding a protein with four hydrophobic domains in central and peripheral myelin. J Neurosci 15:5753–5764
Saravanan K, Büssow H, Weiler N, Gieselmann V, Franken S (2007) A spontaneously immortalized Schwann cell line to study the molecular aspects of metachromatic leukodystrophy. J Neurosci Methods 161:223–233
Sango K, Yamanaka S, Hoffmann A, Okuda Y, Grinberg A, Westphal H, McDonald MP, Crawley JN, Sandhoff K, Suzuki K, Proia RL (1995) Mouse models of Tay-Sachs and Sandhoff diseases differ in neurologic phenotype and ganglioside metabolism. Nat Genet 11:170–176
Ghosh P, Dahms NM, Kornfeld S (2003) Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol 4:202–212
Ramakrishnan H, Khalaj Hedayati K, Lüllmann-Rauch R, Wessig C, Fewou SN, Maier H, Goebel HH, Gieselmann V, Eckhardt M (2007) Increasing sulfatide synthesis in myelin-forming cells of arylsulfatase A-deficient mice causes demyelination and neurological symptoms reminiscent of human metachromatic leukodystrophy. J Neurosci 27:9482–9490
Fewou SN, Büssow H, Schaeren-Wiemers N, Vanier MT, Macklin WB, Gieselmann V, Eckhardt M (2005) Reversal of non-hydroxy:alpha-hydroxy galactosylceramide ratio and unstable myelin in transgenic mice overexpressing UDP-galactose:ceramide galactosyltransferase. J Neurochem 94:469–481
Klein D, Büssow H, Fewou SN, Gieselmann V (2005) Exocytosis of storage material in a lysosomal disorder. Biochem Biophys Res Commun 327:663–667
Simons K, Gruenberg J (2000) Jamming the endosomal system: lipid rafts and lysosomal storage diseases. Trends Cell Biol 10:459–462
Lingwood D, Fisher LJ, Callahan JW, Ballantyne JS (2004) Sulfatide and Na+-K+-ATPase: a salinity-sensitive relationship in the gill basolateral membrane of rainbow trout. J Membr Biol 201:77–84
Lingwood D, Harauz G, Ballantyne JS (2005) Regulation of fish gill Na(+)-K(+)-ATPase by selective sulfatide-enriched raft partitioning during seawater adaptation. J Biol Chem 280:36545–36550
Ramu Y, Xu Y, Lu Z (2006) Enzymatic activation of voltage-gated potassium channels. Nature 442:696–699
Chi S, Qi Z (2006) Regulatory effect of sulphatides on BKCa channels. Br J Pharmacol 149:1031–1038
Buschard K, Blomqvist M, Månsson JE, Fredman P, Juhl K, Gromada J (2006) C16:0 sulfatide inhibits insulin secretion in rat beta-cells by reducing the sensitivity of KATP channels to ATP inhibition. Diabetes 55:2826–2834
Miki T, Seino S (2005) Roles of KATP channels as metabolic sensors in acute metabolic changes. J Mol Cell Cardiol 38:917–925
Soundarapandian MM, Zhong X, Peng L, Wu D, Lu Y (2007) Role of K(ATP) channels in protection against neuronal excitatory insults. J Neurochem 103:1721–1729
Barrier L, Page G, Barc S, Piriou A, Portoukalian J (2003) Sulfatide and GM1 ganglioside modulate the high-affinity dopamine uptake in rat striatal synaptosomes: evidence for the involvement of their ionic charges. Neurochem Int 42:305–313
Hermansson M, Käkelä R, Berghäll M, Lehesjoki AE, Somerharju P, Lahtinen U (2005) Mass spectrometric analysis reveals changes in phospholipid, neutral sphingolipid and sulfatide molecular species in progressive epilepsy with mental retardation, EPMR, brain: a case study. J Neurochem 95:609–617
Ranta S, Zhang Y, Ross B, Lonka L, Takkunen E, Messer A, Sharp J, Wheeler R, Kusumi K, Mole S, Liu W, Soares MB, Bonaldo MF, Hirvasniemi A, de la Chapelle A, Gilliam TC, Lehesjoki AE (1999) The neuronal ceroid lipofuscinoses in human EPMR and mnd mutant mice are associated with mutations in CLN8. Nat Genet 23:233–236
Winter E, Ponting CP (2002) TRAM, LAG1 and CLN8: members of a novel family of lipid-sensing domains? Trends Biochem Sci 27:381–383
Cheng H, Xu J, McKeel DW Jr, Han X (2003) Specificity and potential mechanism of sulfatide deficiency in Alzheimer’s disease: an electrospray ionization mass spectrometric study. Cell Mol Biol (Noisy-le-grand) 49:809–818
Han X, Holtzman DM, McKeel DW Jr, Kelley J, Morris JC (2002) Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: potential role in disease pathogenesis. J Neurochem 82:809–818
Han X, Fagan AM, Cheng H, Morris JC, Xiong C, Holtzman DM (2003) Cerebrospinal fluid sulfatide is decreased in subjects with incipient dementia. Ann Neurol 54:115–119
Strittmatter WJ, Roses AD (1996) Apolipoprotein E and Alzheimer’s disease. Annu Rev Neurosci 19:53–77
Zöller I, Büssow H, Gieselmann V, Eckhardt M (2005) Oligodendrocyte specific ceramide galactosyltransferase (CGT) expression phenotypically rescues CGT deficient mice and demonstrates that CGT activity does not limit brain galactosylceramide level. Glia 52:190–198
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eckhardt, M. The Role and Metabolism of Sulfatide in the Nervous System. Mol Neurobiol 37, 93–103 (2008). https://doi.org/10.1007/s12035-008-8022-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-008-8022-3