Abstract
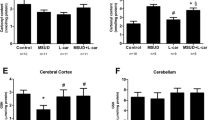
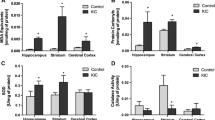
Maple syrup urine disease is an inherited metabolic disease predominantly characterized by neurological dysfunction. However, the mechanisms underlying the neuropathology of this disease are still not defined. Therefore, the aim of this study was to investigate the effect of acute and chronic administration of a branched-chain amino acids (BCAA) pool (leucine, isoleucine, and valine) on acetylcholinesterase (AChE) activity and gene expression in the brain and serum of rats and to assess if antioxidant treatment prevented the alterations induced by BCAA administration. Our results show that the acute administration of a BCAA pool in 10- and 30-day-old rats increases AChE activity in the cerebral cortex, striatum, hippocampus, and serum. Moreover, chronic administration of the BCAA pool also increases AChE activity in the structures studied, and antioxidant treatment prevents this increase. In addition, we show a significant decrease in the mRNA expression of AChE in the hippocampus following acute administration in 10- and 30-day-old rats. On the other hand, AChE expression increased significantly after chronic administration of the BCAA pool. Interestingly, the antioxidant treatment was able to prevent the increased AChE activity without altering AChE expression. In conclusion, the results from the present study demonstrate a marked increase in AChE activity in all brain structures following the administration of a BCAA pool. Moreover, the increased AChE activity is prevented by the coadministration of N-acetylcysteine and deferoxamine as antioxidants.






Similar content being viewed by others
References
Chuang DT, Shih VE (2001) Maple syrup urine disease (branched-chain ketoaciduria). In: Scriver CR, Beaudt AL, Sly WL, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 1971–2005
Mackenzie DY, Woolf LI (1959) Maple syrup urine disease: an inborn error of the metabolism of valine, leucine, and isoleucine associated with gross mental deficiency. Br Med J 1:90–91
Zinnanti WJ, Lazovic J, Griffin K, Skvorak KJ, Paul HS, Homanics GE, Bewley MC, Cheng KC, Lanoue KF, Flanagan JM (2009) Dual mechanism of brain injury and novel treatment strategy in maple syrup urine disease. Brain 132:903–918
Morton DH, Strauss KA, Robinson DL, Puffenberger EG, Kelley RI (2002) Diagnosis and treatment of maple syrup disease: a study of 36 patients. Pediatrics 109:999–1008
Schonberger S, Schweiger B, Schwahn B, Schwarz M, Wendel U (2004) Dysmyelination in the brain of adolescents and young adults with maple syrup urine disease. Mol Genet Metab 82:69–75
Tribble D, Shapira R (1983) Myelin proteins: degradation in rat brain initiated by metabolites causative of maple syrup urine disease. Biochem Biophys Res Commun 114:440–446
Snyderman SE, Norton PM, Roitman E, Holt LE Jr (1964) Maple syrup urine disease, with particular reference to dietotherapy. Pediatrics 34:454–472
Danner DJ, Elsas LJ (1989) Disorders of branched chain amino acid and keto acid metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic basis of inherited disease. McGraw-Hill, New York, pp 671–692
Halestrap AP, Brand MD, Denton RM (1974) Inhibition of mitochondrial pyruvate transport by phenylpyruvate and a-ketoisocaproate. Biochem Biophys Acta 367:102–108
Howell RK, Lee M (1963) Influence of a-keto acids on the respiration of brain in vitro. Proc Soc Exp Biol Med 113:660–663
Land JM, Mowbray J, Clark JB (1976) Control of pyruvate and beta-hydroxybutyrate utilization in rat brain mitochondria and its relevance to phenylketonuria and maple syrup urine disease. J Neurochem 26:823–830
Pilla C, Cardozo RF, Dutra-Filho CS, Wyse AT, Wajner M, Wannmacher CM (2003) Creatine kinase activity from rat brain is inhibited by branched-chain amino acids in vitro. Neurochem Res 28:675–679
Araújo P, Wassermann GF, Tallini K, Furlanetto V, Vargas CR, Wannmacher CM, Dutra-Filho CS, Wyse AT, Wajner M (2001) Reduction of large neutral amino acid levels in plasma and brain of hyperleucinemic rats. Neurochem Int 38:529–537
Wajner M, Vargas CR (1999) Reduction of plasma concentrations of large neutral amino acids in patients with maple urine disease during crises. Arch Dis Child 80:579
Wajner M, Coelho DM, Barschak AG, Araújo PR, Pires RF, Lulhier FL, Vargas CR (2000) Reduction of large neutral amino acid concentration in plasma and CSF of patients with maple syrup urine disease during crises. J Inherit Metab Dis 23:505–512
Appel SH (1966) Inhibition of brain protein synthesis: an approach to a biochemical basis of neurological dysfunction in the amino-acidurias. Trans N Y Acad Sci 29:63–70
Taketomi T, Kunishita T, Hara A, Mizushima S (1983) Abnormal protein and lipid compositions of the cerebral myelin of a patient with maple syrup urine disease. Jpn J Exp Med 53:109–116
Treacy E, Clow CL, Reade TR, Chitayat D, Mamer OA, Scriver CR (1992) Maple syrup urine disease: interrelationship between branched-chain amino-, oxo- and hydroxyacids; implications for treatment; associations with CNS dysmyelination. J Inherit Metab Dis 15:121–135
Blokland A (1995) Acetylcholine: a neurotransmitter for learning and memory? Brain Res 21:285–300
Fodale V, Quattrone D, Trecroci C, Caminiti V, Santamaria LB (2006) Alzheimer’s disease and anaesthesia: implications for the central cholinergic system. Br J Anaesth 97:445–452
Bartus RT, Dean RL 3rd, Beer B, Lippa AS (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217:408–414
Mesulam MM (2004) The cholinergic innervation of the human cerebral cortex. Prog Brain Res 145:67–78
Das A, Dikshit M, Nath C (2001) Profile of acetylcholinesterase in brain areas of male and female rats of adult and old age. Life Sci 68:1545–1555
Descarries L, Gisiger V, Steriade M (1997) Diffuse transmission by acetylcholine in the CNS. Prog Neurobiol 53:603–625
Soreq H, Seidman S (2001) Acetylcholinesterase—new roles for an old actor. Nat Rev Neurosci 2:294–302
Ballard CG, Greig NH, Guillozet-Bongaarts AL, Enz A, Darvesh S (2005) Cholinesterases: roles in the brain during health and disease. Curr Alzheimer Res 2:307–318
Metz CN, Tracey KJ (2005) It takes nerve to dampen inflammation. Nat Immunol 6:756–757
Zimmerman G, Soreq H (2006) Termination and beyond: acetylcholinesterase as a modulator of synaptic transmission. Cell Tissue Res 326:655–669
Silman I, Sussman JL (2005) Acetylcholinesterase: ‘classical’ and ‘non-classical’ functions and pharmacology. Curr Opin Pharmacol 5:293–302
Aruoma OI, Halliwell B, Hoey BM, Butler J (1989) The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med 6:593–597
Moldeus P, Cotgreave IA, Berggren M (1986) Lung protection by a thiol-containing antioxidant: N-acetylcysteine. Respiration 50:31–42
Cotgreave IA (1997) N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Adv Pharmacol 38:205–227
Sprong RC, Winkelhuyzen-Janssen AML, Aarsman CJM, van Oirschot JF, Bruggen T, van Asbeck BS (1998) Low-dose N-acetylcysteine protects rats against endotoxin-mediated oxidative stress, but high-dose increases mortality. Am J Respir Crit Care Med 157:1283–1293
Ritter C, Andrades ME, Reinke A, Menna-Barreto S, Moreira JC, Dal-Pizzol F (2004) Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med 32:342–349
Bridi R, Fontella FU, Pulrolnik V, Braun CA, Zorzi GK, Coelho D, Wajner M, Vargas CR, Dutra-Filho CS (2006) A chemically-induced acute model of maple syrup urine disease in rats for neurochemical studies. J Neurosci Methods 155:224–230
Di-Pietro PB, Dias ML, Scaini G, Burigo M, Constantino L, Machado RA, Dal-Pizzol F, Streck EL (2008) Inhibition of brain creatine kinase activity after renal ischemia is attenuated by N-acetylcysteine and deferoxamine administration. Neurosci Lett 434:139–143
Ellman GI, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Lowry OH, Rosebough NG, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
da Silva RS, Richetti SK, da Silveira VG, Battastini AM, Bogo MR, Lara DR, Bonan CD (2008) Maternal caffeine intake affects acetylcholinesterase in hippocampus of neonate rats. Int J Dev Neuroscience 26:339–343
Amaral AU, Leipnitz G, Fernandes CG, Seminotti B, Schuck PF, Wajner M (2010) Alpha-ketoisocaproic acid and leucine provoke mitochondrial bioenergetic dysfunction in rat brain. Brain Res 1324:75–84
Ribeiro CA, Sgaravatti AM, Rosa RB, Schuck PF, Grando V, Schmidt AL, Ferreira GC, Perry ML, Dutra-Filho CS, Wajner M (2008) Inhibition of brain energy metabolism by the branched-chain amino acids accumulating in maple syrup urine disease. Neurochem Res 33:114–124
Sgaravati AM, Rosa RB, Schuck PF, Ribeiro CAJ, Wannmacher CMD, Wyse ATS, Dutra-Filho CS, Wajner M (2003) Inhibition of brain energy metabolism by the a-keto acids accumulating in maple syrup urine disease. Biochim Biophys Acta 1639:232–238
Barschak AG, Sitta A, Deon M, de Oliveira MH, Haeser A, Dutra-Filho CS, Wajner M, Vargas CR (2006) Evidence that oxidative stress is increased in plasma from patients with maple syrup urine disease. Metab Brain Dis 21:279–286
Bridi R, Araldi J, Sgarbi MB, Testa CG, Durigon K, Wajner M, Dutra-Filho CS (2003) Induction of oxidative stress in rat brain by the metabolites accumulating in maple syrup urine disease. Int J Dev Neurosci 21:327–332
Bridi R, Braun CA, Zorzi GK, Wannmacher CM, Wajner M, Lissi EG, Dutra-Filho CS (2005) Alpha-keto acids accumulating in maple syrup urine disease stimulate lipid peroxidation and reduce antioxidant defences in cerebral cortex from young rats. Met Brain Dis 20:155–167
Bridi R, Latini A, Braun CA, Zorzi GK, Wajner M, Lissi EG, Dutra-Filho CS (2005) Evaluation of the mechanisms involved in leucine-induced oxidative damage in cerebral cortex of young rats. Free Radic Res 39:71–79
Fontella FU, Gassen E, Pulrolni V, Wannmacher CMD, Klein AB, Wajner M, Dutra-Filho CS (2002) Stimulation of lipid peroxidation in vitro in rat brain by the metabolites accumulating in maple syrup urine disease. Metab Brain Dis 17:47–54
Dodd PR, Williams SH, Gundlach AL, Harper PA, Healy PJ, Dennis JA, Johnston GA (1992) Glutamate and gamma-aminobutyric acid neurotransmitter systems in the acute phase of maple syrup urine disease and citrullinemia encephalopathies in newborn calves. J Neurochem 59:582–590
Prensky AL, Moser HW (1967) Changes in the amino acid composition of proteolipids of white matter during maturation of the human nervous system. J Neurochem 14:117–121
Tavares RG, Santos CEF, Tasca C, Wajner M, Souza DO, Dutra-Filho CS (2000) Inhibition of glutamate uptake into synaptic vesicles of rat brain by the metabolites accumulating in maple syrup urine disease. J Neurol Sci 181:44–49
Yudkoff M, Daikhin Y, Lin ZP, Nissim I, Stern J, Pleasure D, Nissin I (1994) Interrelationships of leucine and glutamate metabolism in cultured astrocyts. J Neurochem 62:1192–1202
Mello CF, Feksa L, Brusque AM, Wannmacher CM, Wajner M (1999) Chronic early leucine administration induces behavioral deficits in rats. Life Sci 65:747–55
Tõugu V, Kesvatera T (1996) Role of ionic interactions in cholinesterase catalysis. Biochim Biophys Acta 1298:12–30
Greenfield S (1984) Acetylcholinesterase may have novel functions in the brain. Trends Neurosci 7:364–368
Inestrosa NC, Alvarez A, Pérez CA, Moreno RD, Vicente M, Linker C, Casanueva OI, Soto C, Garrido J (1996) Acetylcholinesterase accelerates assembly of amyloid-b-peptides into Alzheimer’s fibrils: possible role of the peripheral site of the enzyme. Neuron 16:881–891
Karpel R, Sternfeld M, Ginzberg D, Guhl E, Graessmann A, Soreq H (1996) Overexpression of alternative human acetylcholinesterase forms modulates process extensions in cultured glioma cells. J Neurochem 66:114–123
Layer P, Willbold E (1995) Novel functions of cholinesterases in development, physiology and disease. Prog Hisotchem Cytochem 29:1–92
Jiang H, Zhang XJ (2008) Acetylcholinesterase and apoptosis. A novel perspective for an old enzyme. FEBS J 275:612–617
Ben-Ari S, Toiber D, Sas AS, Soreq H, Ben-Shaul Y (2006) Modulated splicing-associated gene expression in P19 cells expressing distinct acetylcholinesterase splice variants. J Neurochem 97:24–34
Park SE, Kim ND, Yoo YH (2004) Acetylcholinesterase plays a pivotal role in apoptosome formation. Cancer Res 64:2652–2655
Jouvet J, Rustin P, Taylor DL, Pocock JM, Felderhoff-Mueser U, Mazarakis ND, Sarraf C, Joashi U, Kozma M, Greenwood K, Edwards AD, Mehmet H (2000) Branched chain amino acids induce apoptosis in neural cells without mitochondrial membrane depolarization or cytochrome c release: implications for neurological impairment associated with maple syrup urine disease. Mol Biol Cell 11:1919–1932
Krishna S, Andersson AM, Semsey S, Sneppen K (2006) Structure and function of negative feedback loops at the interface of genetic and metabolic networks. Nucleic Acids Res 34:2455–2462
Keseler IM, Collado-Vides J, Gama-Castro S, Ingraham J, Paley S, Paulsen IT, Peralta-Gil M, Karp PD (2005) EcoCyc: a comprehensive database resource for Escherichia coli. Nucleic Acids Res 1:334–337
Salgado H, Santos-Zavaleta A, Gama-Castro S, Millan-Zarate D, Diaz-Peredo E, Sanchez-Solano F, Perez-Rueda E, Bonavides-Martinez C, Collado-Vides J, Regulon DB (2001) RegulonDB (version 3.2): transcriptional regulation and operon organization in Escherichia coli K-12. Nucleic Acids Res 29:72–74
Grifman M, Arbel A, Ginzberg D, Glick D, Elgavish S, Shaanan B, Soreq H (1997) In vitro phosphorylation of acetylcholinesterase at non-consensus protein kinase A sites enhances the rate of acetylcholine hydrolysis. Brain Res Mol Brain Res 51:179–87
Aitken RJ, Harkiss D, Knox W, Paterson M, Irvine DS (1998) A novel signal transduction cascade in capacitating human spermatozoa characterized by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J Cell Sci 111:645–656
Herrero MB, Chatterjee S, Lefièvre L, de Lamirande E, Gagnon C (2000) Nitric oxide interacts with the cAMP pathway to modulate capacitation of human spermatozoa. Free Radic Biol Med 29:522–536
Leclerc P, de Lamirande E, Gagnon C (1997) Regulation of protein tyrosine phosphorylation and human sperm capacitation by reactive oxygen species derivatives. Free Radic Biol Med 22:643–656
Aldunate R, Casar JC, Brandan E (2004) Structural and functional organization of synaptic acetylcholinesterase. Brain Res Rev 47:96–104
Melo JB, Agostinho P, Oliveira CR (2003) Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta-peptide. Neurosci Res 45:117–127
Benzi G, Morreti A (1998) Is there a rationale for the use of acetylcholinesterase inhibitors in the therapy of Alzheimer’s disease? Eur J Pharmacol 346:1–13
Das A, Shanker G, Nath C, Pal R, Singh S, Singh HK (2002) A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba: anticholinesterase and cognitive enhancing activities. Pharmacol Biochem Behav 73:893–900
Grisaru D, Sternfeld M, Eldor A, Glick D, Soreq H (1999) Structural roles of acetylcholinesterase variants in biology and pathology. Eur J Biochem 264:672–686
Kaur T, Pathak CM, Pandhi P, Khanduja KL (2007) Effects of green tea extract on learning, memory, behavior and acetylcholinesterase activity in young and old male rats. Brain Cogn 67:25–30
Mesulam MM, Guillozet A, Shaw P, Levey A (2002) Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience 110:627–639
Acknowledgments
This research was supported by grants from Programa de Pós-graduação em Ciências da Saúde—Universidade do Extremo Sul Catarinense and Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scaini, G., de Rochi, N., Jeremias, I.C. et al. Evaluation of Acetylcholinesterase in an Animal Model of Maple Syrup Urine Disease. Mol Neurobiol 45, 279–286 (2012). https://doi.org/10.1007/s12035-012-8243-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-012-8243-3