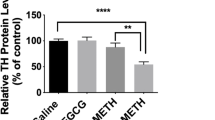
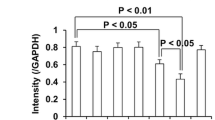
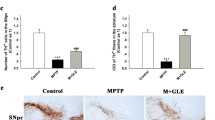
Abstract
Ginsenoside Re, one of the main constituents of Panax ginseng, possesses novel antioxidant and anti-inflammatory properties. However, the pharmacological mechanism of ginsenoside Re in dopaminergic degeneration remains elusive. We suggested that protein kinase C (PKC) δ mediates methamphetamine (MA)-induced dopaminergic toxicity. Treatment with ginsenoside Re significantly attenuated methamphetamine-induced dopaminergic degeneration in vivo by inhibiting impaired enzymatic antioxidant systems, mitochondrial oxidative stress, mitochondrial translocation of protein kinase Cδ, mitochondrial dysfunction, pro-inflammatory microglial activation, and apoptosis. These protective effects were comparable to those observed with genetic inhibition of PKCδ in PKCδ knockout (−/−) mice and with PKCδ antisense oligonucleotides, and ginsenoside Re did not provide any additional protective effects in the presence of PKCδ inhibition. Our results suggest that PKCδ is a critical target for ginsenoside Re-mediated protective activity in response to dopaminergic degeneration induced by MA.











Similar content being viewed by others
References
Fitzmaurice PS, Tong J, Yazdanpanah M, Liu PP, Kalasinsky KS, Kish SJ (2006) Levels of 4-hydroxynonenal and malondialdehyde are increased in brain of human chronic users of methamphetamine. J Pharmacol Exp Ther 319:703–709
Guilarte TR (2001) Is methamphetamine abuse a risk factor in parkinsonism? Neurotoxicology 22:725–731
Kita T, Wagner GC, Nakashima T (2003) Current research on methamphetamine-induced neurotoxicity: animal models of monoamine disruption. J Pharmacol Sci 92:178–195
Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ (1996) Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 2:699–703
Wilson JM, Levey AI, Rajput A, Ang L, Guttman M, Shannak K, Niznik HB, Hornykiewicz O, Pifl C, Kish SJ (1996) Differential changes in neurochemical markers of striatal dopamine nerve terminals in idiopathic Parkinson’s disease. Neurology 47:718–726
Zhong XH, Haycock JW, Shannak K, Robitaille Y, Fratkin J, Koeppen AH, Hornykiewicz O, Kish SJ (1995) Striatal dihydroxyphenylalanine decarboxylase and tyrosine hydroxylase protein in idiopathic Parkinson’s disease and dominantly inherited olivopontocerebellar atrophy. Mov Disord 10:10–17
Kim HC, Jhoo WK, Shin EJ, Bing G (2000) Selenium deficiency potentiates methamphetamine-induced nigral neuronal loss; comparison with MPTP model. Brain Res 862:247–252
Sonsalla PK, Jochnowitz ND, Zeevalk GD, Oostveen JA, Hall ED (1996) Treatment of mice with methamphetamine produces cell loss in the substantia nigra. Brain Res 738:172–175
Lu JM, Yao Q, Chen C (2009) Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol 7:293–302
Joo KM, Lee JH, Jeon HY, Park CW, Hong DK, Jeong HJ, Lee SJ, Lee SY, Lim KM (2010) Pharmacokinetic study of ginsenoside Re with pure ginsenoside Re and ginseng berry extracts in mouse using ultra performance liquid chromatography/mass spectrometric method. J Pharm Biomed Anal 51:278–283
Xie JT, Shao ZH, Hoek TLV, Chang WT, Li J, Mehendale S, Wang CZ, Hsu CW, Becker LB, Yin JJ, Yuan XS (2006) Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur J Pharmacol 532:201–207
Ko SK, Bae HM, Cho OS, Im BO, Chung SH, Lee BY (2008) Analysis of ginsenoside composition of ginseng berry and seed. Food Sci Biotechnol 17:1379–1382
Ko SK, Cho OS, Bae HM, Im BO, Lee OH, Lee BY (2011) Quantitative analysis of ginsenosides composition in flower buds of various ginseng plants. J Korean Soc Appl Biol Chem 54:154–157
Bai CX, Sunami A, Namiki T, Sawanobori T, Furukawa T (2003) Electrophysiological effects of ginseng and ginsenoside Re in guinea pig ventricular myocytes. Eur J Pharmacol 476:35–44
Bai CX, Takahashi K, Masumiya H, Sawanobori T, Furukawa T (2004) Nitric oxide-dependent modulation of the delayed rectifier K+ current and the L-type Ca2+ current by ginsenoside Re, an ingredient of Panax ginseng, in guinea-pig cardiomyocytes. Br J Pharmacol 142:567–575
Kim HS, Lee JH, Goo YS, Nah SY (1998) Effects of ginsenosides on Ca2+ channels and membrane capacitance in rat adrenal chromaffin cells. Brain Res Bull 46:245–251
Kim KH, Song K, Yoon SH, Shehzad O, Kim YS, Son JH (2012) Rescue of PINK1 protein null-specific mitochonsdrial complex IV deficits ginsenoside Re activation of nitric oxide signaling. J Biol Chem 287:44109–44120
Xu BB, Liu CQ, Gao X, Zhang WQ, Wang SW, Cao YL (2005) Possible mechanisms of the protection of ginsenoside Re against MPTP-induced apoptosis in substantia nigra of Parkinson’s disease mouse model. J Asian Nat Prod Res 7:215–224
Nishizuka Y (1986) Studies and perspectives of protein kinase C. Science 233:305–312
Nishizuka Y (1992) Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258:607–614
Basu A, Pal D (2010) Two faces of protein kinase Cδ: the contrasting roles of PKCδ in cell survival and cell death. Sci World J 10:2272–2284
Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V (2003) Role of proteolytic activation of PKCδ in oxidative stress-induced apoptosis. Antioxid Redox Signal 5:609–620
Kaul S, Kanthasamy A, Kitazawa M, Anantharam V, Kanthasamy AG (2003) Caspase-3 dependent proteolytic activation of protein kinase Cδ mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration. Eur J Neurosci 18:1387–1401
Zhang D, Kanthasamy A, Anantharam V (2011) Effects of manganese on tyrosine hydroxylase (TH) activity and TH-phosphorylation in a dopaminergic neural cell line. Toxicol Appl Pharmacol 254:65–71
Shin EJ, Duong CX, Nguyen TX, Bing G, Bach JH, Park DH, Nakayama K, Ali SF, Kanthasamy AG, Cadet JL, Nabeshima T, Kim HC (2011) PKCδ inhibition enhances tyrosine hydroxylase phosphorylation in mice after methamphetamine treatment. Neurochem Int 59:39–50
Shin EJ, Duong CX, Nguyen XKT, Li Z, Bing G, Bach JH, Park DH, Nakayama K, Ali SF, Kanthasamy AG, Cadet JL, Nabeshima T, Kim HC (2012) Role of oxidative stress in methamphetamine-induced dopaminergic toxicity mediated by protein kinase Cδ. Behav Brain Res 232:98–113
Shin EJ, Nabeshima T, Suh HW, Jhoo WK, Oh KW, Lim YK, Kim DS, Choi KH, Kim HC (2005) Ginsenosides attenuate methamphetamine-induced behavioral side effects in mice via activation of adenosine A2A receptors: possible involvements of the striatal reduction in AP-1 DNA binding activity and proenkephalin gene expression. Behav Brain Res 158:143–157
Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, Tsukiyama T, Nagahama H, Ohno S, Hatakeyama S, Nakayama KI (2002) Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cδ. Nature 416:865–869
Zhu JP, Xu W, Angulo JA (2006) Methamphetamine-induced cell death: selective vulnerability in neuronal subpopulations of the striatum in mice. Neuroscience 140:607–622
Zhu JP, Xu W, Angulo N, Angulo JA (2006) Methamphetamine-induced striatal apoptosis in the mouse brain: comparison of a binge to an acute bolus drug administration. Neurotoxicology 27:131–136
Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, Subbulakshmi V, Feldman GM, Wientjes FB, Cathcart MK (2004) Protein kinase Cδ required for p47phox phosphorylation and translocation in activated human monocytes. J Immunol 173:5730–5738
Khodjakov A, Lizunova EM, Minin AA, Koonce MP, Gyoeva FK (1998) A specific light chain of kinesin associates with mitochondria in cultured cells. Mol Biol Cell 9:333–343
Xiong Y, Gu Q, Peterson PL, Muizelaar JP, Lee CP (1997) Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J Neurotrauma 14:23–34
Shin EJ, Jeong JH, Kim AY, Koh YH, Nah SY, Kim WK, Ko KH, Kim HJ, Wie MB, Kwon YS, Yoneda Y, Kim HC (2009) Protection against kainate neurotoxicity by ginsenosides: attenuation of convulsive behavior, mitochondrial dysfunction and oxidative stress. J Neurosci Res 87:710–722
Jhoo JH, Kim HC, Nabeshima T, Yamada K, Shin EJ, Jhoo WK, Kim W, Kang KS, Jo SA, Woo JI (2004) Beta-amyloid (1–42)-induced learning and memory deficits in mice: involvement of oxidative burdens in the hippocampus and cerebral cortex. Behav Brain Res 155:185–196
Aebi H (1984) Catalase in vitro. In: Abelon JN, Simon MI (eds) Methods in enzymology. Academic, New York, pp 121–126
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71:952–958
Wang Q, Shin EJ, Nguyen XKT, Li Q, Bach JH, Bing G, Kim WK, Kim HC, Hong JS (2012) Endogenous dynorphin protects against neurotoxin-elicited nigrostriatal dopaminergic neuron damage and motor deficits in mice. J Neuroinflammation 9:124
West MJ (1993) New stereological methods for counting neurons. Neurobiol Aging 14:275–285
Shin EJ, Jeong JH, Bing G, Park ES, Chae JS, Yen TPH, Kim WK, Wie MB, Jung BD, Kim HJ, Lee SY, Kim HC (2008) Kainate-induced mitochondrial oxidative stress contributes to hippocampal degeneration in senescence-accelerated mice. Cell Signal 20:645–658
Tran HY, Shin EJ, Saito K, Nguyen XK, Chung YH, Jeong JH, Bach JH, Park DH, Yamada K, Nabeshima T, Yoneda Y, Kim HC (2012) Protective potential of IL-6 against trimethyltin-induced neurotoxicity in vivo. Free Radic Biol Med 52:1159–1174
Oliver CN, Ahn BW, Moerman EJ, Goldstein S, Stadtman ER (1987) Age-related changes in oxidized proteins. J Biol Chem 262:5488–5491
Bruce-Keller AJ, Geddes JW, Knapp PE, McFall RW, Keller JN, Holtsberg FW, Parthasarathy S, Steiner SM, Mattson MP (1999) Anti-death properties of TNF against metabolic poisoning: mitochondrial stabilization by MnSOD. J Neuroimmunol 93:53–71
Qu M, Zhou Z, Xu S, Chen C, Yu Z, Wang D (2011) Mortalin overexpression attenuates beta-amyloid-induced neurotoxicity in SH-SY5Y cells. Brain Res 1367:336–345
Matton MP, Keller JN, Begley JC (1998) Evidence for synaptic apoptosis. Exp Neurol 153:35–48
Xu S, Pi H, Chen Y, Zhang N, Guo P, Lu Y, He M, Xie J, Zhong M, Zhang Y, Yu Z, Zhou Z (2013) Cadmium induced Drp1-dependent mitochondrial fragmentation by disturbing calcium homeostasis in its hepatotoxicity. Cell Death Dis 4:e540
Nagatsu T, Oka K, Kato T (1979) Highly sensitive assay for tyrosine hydroxylase activity by high-performance liquid chromatography. J Chromatogr 163:247–252
Chance B, Sies H, Boveris A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59:527–605
Xiong Y, Shie FS, Zhang J, Lee CP, Ho YS (2004) The protective role of cellular glutathione peroxidase against trauma-induced mitochondrial dysfunction in the mouse brain. J Stroke Cerebrovasc Dis 13:129–137
Mari M, Morales A, Colell A, Garcia-Ruiz C, Kaplowitz N, Fernadez-Checa JC (2013) Mitochondrial glutathione: features, regulation and role in disease. Biochim Biophys Acta 1830:3317–3328
Prohaska JR, Ganther HE (1976) Selenium and glutathione peroxidase in developing rat brain. J Neurochem 27:1379–1387
Liccione JJ, Maines MD (1988) Selective vulnerability of glutathione metabolism and cellular defense mechanisms in rat striatum to manganese. J Pharmacol Exp Ther 247:156–161
Mattson MP (2012) Parkinson’s disease: don’t mess with calcium. J Clin Invest 122:1195–1198
Kuwabara T, Imajoh-Ohmi S (2004) LPS-induced apoptosis is dependent upon mitochondrial dysfunction. Apoptosis 9:467–474
Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM (2004) Methamphetamine neurotoxicity in dopamine nerve endings of striatum is associated with microglial activation. J Pharmacol Exp Ther 311:1–7
Thomas DM, Kuhn DM (2005) Attenuated microglial activation mrdiates tolerance to the neurotoxic effects of methamphetamine. J Neurochem 92:790–797
Kuhn DM, Francescutti-Verbeem DM, Thomas DM (2008) Dopamine disposition in the presynaptic process regulates the severity of methamphetamine-induced neurotoxicity. Ann NY Acad Sci 1139:118–126
Friend DM, Keefe KA (2013) Glial reactivity in resistance to methamphetamine-induced neurotoxicity. J Neurochem 125:566–574
Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG (2003) Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol 462:223–240
Flemming JC, Norenberg MD, Ramsay DA, Dekban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC (2006) The cellular inflammatory response in human spinal cords injury. Brain 129:3249–3269
Gordon S (2003) Alternative activation of macrophage. Nat Rev Immunol 3:23–35
Gordon S (2007) Macrophage heterogeneity and tissue lipids. J Clin Invest 117:89–93
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686
Deng X, Cadet JL (2000) Methamphetamine-induced apoptosis is attenuated in the strata of copper-zinc superoxide dismutase transgenic mice. Mol Brain Res 83:121–124
Kuroda KO, Ornthanalai VG, Kato T, Murphy NP (2010) FosB null mutant mice show enhanced methamphetamine neurotoxicity: potential involvement of FosB in intracellular feed back signaling and astroglial function. Neuropsychopharmacology 35:641–655
Walsh SL, Wagner GC (1992) Motor impairments after methamphetamine-induced neurotoxicity in the rat. J Pharmacol Exp Ther 263:617–626
Kim HC, Shin EJ, Jang CG, Lee MK, Eun JS, Hong JT, Oh KW (2005) Pharmacological action of Panax ginseng on the behavioral toxicities induced by psychotropic agents. Arch Pharm Res 28:995–1001
Oh KW, Kim HS, Wagner GC (1997) Ginseng total saponin inhibits the dopaminergic depletions induced by methamphetamine. Planta Med 63:80–81
Wu CF, Liu YL, Song M, Liu W, Wang JH, Li X, Yang JY (2003) Protective effects of pseudoginsenoside-F11 on methamphetamine-induced neurotoxicity in mice. Pharmacol Biochem Behav 76:103–109
Shin EJ, Koh YH, Kim AY, Nah SY, Jeong JH, Chae JS, Kim SC, Yen TPH, Yoon HJ, Kim WK, Ko KH, Kim HC (2009) Ginsenosides attenuate kainic acid-induced synaptosomal oxidative stress via stimulation of adenosine A2A receptors in rat hippocampus. Behav Brain Res 197:239–245
Lopez MVN, Cuadrado MPGS, Ruiz-Poveda OMP, Del Frseno AMV, Acccame MEC (2007) Neuroprotective effect of individual ginsenosides on astrocytes primary culture. Biochim Biophys Acta 1770:1308–1316
Paul S, Shin HP, Kang SC (2012) Inhibition of inflammations and macrophage activated by ginsenoside-Re isolated from Korean ginseng (Panax ginseng C.A. Meyer). Food Chem Toxicol 50:1354–1361
Carter CA (2000) Protein kinase C as a drug target: implications for drug or diet prevention and treatment of cancer. Curr Drug Targets 1:163–183
Goldberg M, Steinberg SF (1996) Tissue-specific developmental regulation of protein kinase C isoforms. Biochem Pharmacol 51:1089–1093
Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM (2003) Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol 157:1015–1022
Callaghan RC, Cunningham JK, Sajeev G, Kish SJ (2010) Incidence of Parkinson’s disease among hospital patients with methamphetamine-use disorders. Mov Disord 25:2333–2339
Callaghan RC, Cunningham JK, Sykes J, Kish SJ (2012) Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend 120:35–40
Coyle JT, Puttfarcken P (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science 262:689–694
Halliwell B (1992) Reactive oxygen species and the central nervous system. J Neurochem 59:1609–1623
Hirata H, Ladenheim B, Rothman RB, Epstein C, Cadet JL (1995) Methamphetamine-induced serotonin neurotoxicity is mediated by superoxide radicals. Brain Res 677:345–347
Kim HC, Jhoo WK, Choi DY, Im DH, Shin EJ, Suh JH, Floyd RA, Bing G (1999) Protection of methamphetamine nigrostriatal toxicity by dietary selenium. Brain Res 851:76–86
Floyd RA, Carney JM (1992) Free radical damage to protein and DNA: mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann Neurol 32:S22–S27
Asanuma M, Miyazaki I, Ogawa N (2003) Dopamine- or L-DOPA-induced neurotoxicity: the role of dopamine quinine formation and tyrosinase in a model of Parkinson’s disease. Neurotox Res 5:165–176
Nicholls DG (2009) Mitochondrial calcium function and dysfunction in the central nervous system. Biochim Biopys Acta 1787:1416–1424
Ryu JK, Nagai A, Kim J, Lee MC, McLarnon JC, Kim SU (2003) Microglial activation and cell death induced by the mitochondrial toxin 3-nitropropionic acid: in vitro and in vivo studies. Neurobiol Dis 12:121–132
Hald A, Lotharius J (2005) Oxidative stress and inflammation in Parkinson’s disease: is there a causal link? Exp Neurol 193:279–290
Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ukei T, Mori N, Gold MS, Cadet JL (2008) Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci 28:5756–5761
Kitamura O, Takeichi T, Wang EL, Tokunaga I, Ishigami A, Kubo S (2010) Microglial and astrocytic changes in the striatum of methamphetamine abusers. Leg Med 12:57–62
Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, Cano J, Brundin P, Englund E, Venero JL, Joseph B (2011) Caspase signaling controls microglia activation and neurotoxicity. Nature 472:319–325
O’Callaghan JP, Miller DB (1994) Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mice. J Pharmacol Exp Ther 270:741–751
Nakajima A, Yamada K, Nagai T, Uchiyama T, Miyamoto Y, Mamiya T, He J, Nitta A, Mizuno M, Tran MH, Seto A, Yoshimura M, Kitaichi K, Hasegawa T, Saito K, Yamada Y, Seishima M, Sekikawa K, Kim HC, Nabeshima T (2004) Role of tumor necrosis factor-alpha in methamphetamine-induced drug dependence and neurotoxicity. J Neurosci 24:2212–2225
Kawasaki T, Ishihara K, Ago Y, Nakamura S, Itoh S, Baba A, Matsuda T (2006) Protective effect of the radical scavenger edaravone against methamphetamine-induced dopaminergic neurotoxicity in mouse striatum. Eur J Pharmacol 542:92–99
Jung BD, Shin EJ, Nguyen XK, Jin CH, Bach JH, Park SJ, Nah SY, Wie MB, Bing G, Kim HC (2010) Potentiation of methamphetamine neurotoxicity by intrastriatal lipopolysaccharide administration. Neurochem Int 56:229–244
Lenard LG, Beer B (1975) Relationship of brain levels of norepinephrine and dopamine to avoidance behavior in rats after intraventricular administration of 6-hydoxydopamine. Pharmacol Biochem Behav 3:895–899
Miyazaki I, Asanuma M, Kikkawa Y, Takeshima M, Murakami S, Miyoshi K, Sogawa N, Kita T (2011) Astrocyte-derived metallothionein protects dopaminergic neurons from dopamine quinone toxicity. Glia 59:435–451
Zhang W, Shin EJ, Wang T, Lee PH, Pang H, Wie MB, Kim WK, Kim SJ, Huang WH, Wang W, Zhang W, Hong JS, Kim HC (2006) Hydroxymorphinan, a metabolite of dextromethorphan, protects nigrostriatal pathway against MPTP-elicited damage both in vivo and in vitro. FASEB J 20:2496–2511
Attele AS, Wu JA, Yuan CS (1999) Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol 58:1685–1693
Kim YK, Yoo DS, Xu H, Park NI, Kim HH, Choi JE, Park SU (2009) Ginsenoside content of berries and roots of three typical Korean ginseng (Panax ginseng) cultivars. Nat Prod Commun 4:903–906
Acknowledgments
This study was supported by a grant (no. 110113-3) from the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (IPET), Republic of Korea. Thuy-Ty Lan Nguyen was supported by BK21 PLUS project. The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/ECwpMn and http://www.textcheck.com/certificate/01ZHRL.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shin, EJ., Shin, S.W., Nguyen, TT.L. et al. Ginsenoside Re Rescues Methamphetamine-Induced Oxidative Damage, Mitochondrial Dysfunction, Microglial Activation, and Dopaminergic Degeneration by Inhibiting the Protein Kinase Cδ Gene. Mol Neurobiol 49, 1400–1421 (2014). https://doi.org/10.1007/s12035-013-8617-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8617-1