Abstract
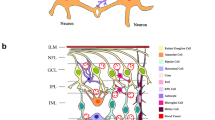
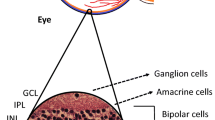
Diabetic retinopathy (DR) was earlier recognized as a vascular disease, but nowadays, it is considered as a neurovascular disorder. Neuronal death is the primary change which leads to various vascular changes which are visible to an ophthalmologist. But these changes are feature of an advanced disease and can affect vision at any moment of time. There are various evidences which suggests that glutamate excitotoxicity, hyperhomocysteinemia, kynurenic acid, and erythro-poietin plays important role in causation of retinal ganglionic cell apoptosis in diabetic patients. Adaptive optics, a new imaging technique, also showed that loss of photoreceptors (specialized neurons) is the early change in diabetic retinopathy. These changes suggest DR as a neurovascular disorder. Neuroprotective agents also showed good results in delaying progression of DR especially memantine, insulin receptor activation, and neurotrophic factors. More research in this field will help us to find novel therapeutic measures for DR, which can delay or even stop progression of DR at a very early stage.
Similar content being viewed by others
References
Ramachandran A, Das A, Joshi S, Yajnik C, Shah S, Prasanna K (2010) Current status of diabetes in India and need for novel therapeutic agents. Suppl JAPI 58:7–9
Simó R, Hernández C (2009) Advances in the medical treatment of diabetic retinopathy. Diabetes Care 32:1556–1562
Hernández C, Simó R (2007) Strategies for blocking angiogenesis in diabetic retinopathy by intravitreal therapy. From basic science to clinical practice. Exp Opin Investig Drug 16:1209–1226
Simó R, Hernández C (2008) Intravitreous anti-VEGF for diabetic retinopathy: hopes and fears for a new therapeutic strategy. Diabetologia 51:1574–1580
Barber AG (2003) A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry 27:283–290
Asnaghi V, Gerhardinger C, Hoehn T, Adeboje A, Lorenzi M (2003) A role of the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in the rat. Diabetes 52:506–511
Abu-El-Asar AM, Dralands L, Missoten L, Al-Jadaan IA, Geboes K (2004) Expression of apoptosis markers in the retinas of human subjects with diabetes. Invest Ophthalmol Vis Sci 45:2760–2766
Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, Strother JM (1998) Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes 47:815–820
Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW (1998) Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest 102:783–791
Peng PH, Lin HS, Lin S (2009) Nerve fibre layer thinning in patients with preclinical retinopathy. Can J Ophthalmol 44:417–422
Bearse MA, Han Y, Schneck ME, Adams AJ (2004) Retinal function in normal and diabetic eyes mapped with the slow flash multifocal electroretinogram. Invest Ophthalmol Vis Sci 45:296–304
Fletcher EL, Phipps JA, Ward MM, Puthussery T, Wilkinson-Berka JL (2007) Neuronal and glial cell abnormality as predictors of progression of diabetic retinopathy. Curr Pharm Des 13:2699–2712
Mohr S, Xi X, Tang J, Kern TS (2002) Caspase activation in retinas of diabetic and galactosemic mice and diabetic patients. Diabetes 51:1172–1179
Metea MR, Newman EA (2007) Signalling within the neurovascular unit in the mammalian retina. Exp Physiol 92:635–640
Kusari J, Zhou S, Padillo E, Clarke KG, Gil DW (2007) Effect of memantine on neuroretinal function and retinal vascular changes of streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci 48:5152–5159
Kusari J, Zhou SX, Padillo E, Clarke KG, Gil DW (2010) Inhibition of vitreoretinal VEGF elevation and blood-retinal barrier breakdown in streptozotocin-induced diabetic rats by brimonidine. Invest Ophthalmol Vis Sci 51:1044–1051
Feng Y, Wang Y, Stock O, Pfister F, Tanimoto N et al (2009) Vasoregression linked to neuronal damage in the rat with defect of polycystin-2. PLoS One 6:7328
Feng Y, Wang Y, Li L, Wu L, Hoffmann S et al (2011) Gene expression profiling of vasoregression in the retina—involvement of microglial cells. PLoS One 6:16865
Kern TS, Du Y, Miller CM, Hatala DA, Levin LA (2010) Overexpression of Bcl-2 in vascular endothelium inhibits the microvascular lesions of diabetic retinopathy. Am J Pathol 176:2550–2558
Martin PM, Roon P, van Ells TK, Ganapathy V, Smith SB (2004) Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci 45:3330–3336
Simó R, Lecube A, Sararols L, García-Arumí J, Segura RM, Casamitjana R et al (2002) Deficit of somatostatin-like immunoreactivity in the vitreous fluid of diabetic patients: possible role in the development of proliferative diabetic retinopathy. Diabetes Care 25:2282–2286
Hernández C, Carrasco E, Casamitjana R, Deulofeu R, García-Arumí J, Simó R (2005) Somatostatin molecular variants in the vitreous fluid: a comparative study between diabetic patients with proliferative diabetic retinopathy and nondiabetic control subjects. Diabetes Care 28:1941–1947
Carrasco E, Hernández C, de Torres I, Farres J, Simó R (2008) Lowered cortistatin expression is an early event in the human diabetic retina and is associated with apoptosis and glial activation. Mol Vis 14:1496–1502
García-Ramírez M, Canals F, Hernández C, Colomé N, Ferrer C, Carrasco E et al (2007) Proteomic analysis of human vitreous fluid by DIGE: a new strategy for identifying potential candidates in the pathogenesis of proliferative diabetic retinopathy. Diabetologia 50:1294–1303
Garcia-Ramírez M, Hernández C, Villarroel M, Canals F, Alonso MA, Fortuny R et al (2009) Interphotoreceptor retinoid-binding protein (IRBP) is downregulated at early stages of diabetic retinopathy. Diabetologia 52:2633–2641
Hernández C, Fonollosa A, García-Ramírez M, Higuera M, Catalán R, Miralles A et al (2006) Erythropoietin is expressed in the human retina and it is highly elevated in the vitreous fluid of patients with diabetic macular edema. Diabetes Care 29:2028–2033
García-Ramírez M, Hernández C, Simó R (2008) Expression of erythropoietin and its receptor in the human retina: a comparative study of diabetic and nondiabetic subjects. Diabetes Care 31:1189–1194
Brafman A, Mett I, Shafir M, Gottlieb H, Damari G, Gozlan-Kelner T (2004) Invest Ophthalmol Vis Sci 45:3796
Ng YK, Zeng XX, Ling EA (2004) Expression of glutamate receptors and calcium-binding proteins in the retina of streptozotocin-induced diabetic rats. Brain Res 1018(20):66–72
Silva KC, Rosales MA, Biswas SK, Lopes de Faria JB, Lopes de Faria JM (2009) Diabetic retinal neurodegeneration is associated with mitochondrial oxidative stress and is improved by angiotensin receptor blocker in a model that combines hypertension and diabetes. Diabetes 58:1382–1390
Zong H, Ward M, Madden A, Yong PH, Limb GA, Curtis TM et al (2010) Hyperglycemia-induced pro-inflammatory responses by retinal Müller glia are regulated by the receptor for advanced glycation end-products (RAGE). Diabetologia 53:2565–2566
Ganapathy PS, White RE, Ha Y, Bozard BR, McNeil PL, Caldwell RW, Kumar S, Black SM, Smith SB et al (2011) The role of N-methyl-D-aspartate receptor activation in homocysteine-induced death of retinal ganglion cells. Invest Ophthalmol Vis Sci 52:5515–5524
Lipton SA, Kim WK, Choi YB, Kumar S, D’Emilia DM et al (1997) Neurotoxicity associated with dual actions of homocysteine at the N-methyl-d-aspartate receptor. Proc Natl Acad Sci U S A 94:5923–5928
Chmiel-Perzyńska I, Perzyński A, Wielosz M, Urbańska EM (2007) Hyperglycemia enhances the inhibitory effect of mitochondrial toxins and D,L-homocysteine on the brain production of kynurenic acid. Pharmacol Rep 59:268–273
Munipally PK, Agraharm SG, Valavala VK, Gundae S, Turlapati NR (2011) Evaluation of indoleamine 2,3-dioxygenase expression and kynurenine pathway metabolites levels in serum samples of diabetic retinopathy patients. Arch Physiol Biochem 117:254–258
Zwilling D, Huang SY, Sathyasaikumar KV, Notarangelo FM, Guidetti P, Wu HQ et al (2011) Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell 145:863–874
Lombardo M, Serrao S, Devaney N et al (2013) Adaptive optics technology for high-resolution retinal imaging. Sensors (Basel) 13:334–366
Lombardo M, Parravano M, Giuseppe LG, Varano M, Boccassini B et al (2003) Adaptive optics imaging of parafoveal cones in type 1 diabetes. Retina 0:1–12
Sakata K, Funatsu H, Harino S et al (2006) Relationship between macular microcirculation and progression of diabetic macular oedema. Ophthalmology 113:1385–1391
Ozawa S, Kamiya H, Tsuzuki K (1998) Gluatamate receptor in the mammalian nervous system. Prog Neurobiol 54:581
Ferreira IL, Duarte CB, Carvalho AP (1998) Kainate-induced retina amacrine-like cell damage is mediated by AMPA receptors. Neuroreport 9:3471–3475
Apoptosis WP (1994) Retinitis pigmentosa, and degeneration. Biochem Cell Biol 72:489–549
Ambati J, Chalam KV, Chawla DK, D’Angio CT, Guillet EG et al (1997) Elevated gamma-aminobutyric acid, glutamate, and vascular endothelial growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch Ophthalmol 115:1161–1166
Gorovits R, Avidan N, Avisar N, Shaked I, Vardimon L (1997) Glutamine synthetase protects against neuronal degeneration in injured retinal tissue. Proc Natl Acad Sci U S A 94:7024–7029
Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S et al (2003) Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 348:1333–1341
Vorwerk CK, Lipton SA, Zurakowski D, Hyman BT, Sabel BA et al (1996) Chronic low-dose glutamate is toxic to retinal ganglion cells. Toxicity blocked by memantine. Invest Ophthalmol Vis Sci 37:1618–1624
Lipton SA (2004) Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. Neurorx 1:101–110
Reiter CE, Gardner TW (2003) Functions of insulin and insulin receptor signaling in retina: possible implications for diabetic retinopathy. Prog Retin Eye Res 22:545–562
Dahl-Jorgensen K, Brinchmann-Hansen O, Hanssen KF, Sandvik L, Aagenaes O (1985) Rapid tightening of blood glucose control leads to transient deterioration of retinopathy in insulin dependent diabetes mellitus: the Oslo study. Br Med J (Clin Res Ed) 290:811–815
Kondo T, Vicent D, Suzuma K, Yanagisawa M, King GL et al (2003) Knockout of insulin and IGF-1 receptors on vascular endothelial cells protects against retinal neovascularization. J Clin Invest 111:1835–1842
Poulaki V, Qin WY, Joussen AM, Hurlbut P, Wiegand SJ, Rudge J et al (2002) Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin Invest 109:805–815
The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group (2000) Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 342:381
Yoshii A, Constantine-Paton M (2010) Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol 70:304–322
Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S (2010) Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol 70:271–288
Chen H, Weber AJ (2001) BDNF enhances retinal ganglion cell survival in cats with optic nerve damage. Invest Ophthalmol Vis Sci 42:966–974
Lom B, Cogen J, Sanchez AL, Vu T, Cohen-Cory S (2002) Local and target-derived brain-derived neurotrophic factor exert opposing effects on the dendritic arborization of retinal ganglion cells in vivo. J Neurosci 22:7639–7649
Pinzón-Duarte G, Arango-González B, Guenther E, Kohler K (2004) Effects of brain-derived neurotrophic factor on cell survival, differentiation and patterning of neuronal connections and Müller glia cells in the developing retina. Eur J Neurosci 19:1475–1484
Hu Y, Cho S, Goldberg JL (2010) Neurotrophic effect of a novel TrkB agonist on retinal ganglion cells. Invest Ophthalmol Vis Sci 51:1747–1754
Sánchez-Migallón MC, Nadal-Nicolás FM, Jiménez-López M, Sobrado-Calvo P, Vidal-Sanz M, Agudo-Barriuso M (2011) Brain derived neurotrophic factor maintains Brn3a expression in axotomized rat retinal ganglion cells. Exp Eye Res 92:260–267
Dai M, Xia XB, Xiong SQ (2012) BDNF regulates GLAST and glutamine synthetase in mouse retinal Müller cells. J Cell Physiol 227:596–603
Nakagawa T, Ono-Kishino M, Sugaru E, Yamanaka M, Taiji M, Noguchi H (2002) Brain-derived neurotrophic factor (BDNF) regulates glucose and energy metabolism in diabetic mice. Diabetes Metab Res Rev 18:185–191
Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P et al (2007) Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 50:431–438
Yamanaka M, Itakura Y, Ono-Kishino M, Tsuchida A, Nakagawa T et al (2008) Intermittent administration of brain-derived neurotrophic factor (BDNF) ameliorates glucose metabolism and prevents pancreatic exhaustion in diabetic mice. J Biosci Bioeng 105:395–402
Harada C, Harada T, Quah HM, Maekawa F, Yoshida K et al (2003) Potential role of glial cell line-derived neurotrophic factor receptors in Müller glial cells during light induced retinal degeneration. Neuroscience 122:229–235
Azadi S, Johnson LE, Paquet-Durand F, Perez MT, Zhang Y et al (2007) CNTF + BDNF treatment and neuroprotective pathways in the rd1 mouse retina. Brain Res 1129:116–129
Barnstable CJ, Tombran-Tink J (2004) Neuroprotective and antiangiogenic actions of PEDF in the eye: molecular targets and therapeutic potential. Prog Retin Eye Res 23:561–577
Saint-Geniez M, Maharaj ASR, Walshe TE, Tucker BA, Sekiyama E et al (2008) Endogenous VEGF is required for visual function: evidence for a survival role on Müller cells and photoreceptors. PLoS One 3:3554
Humphries S, Kushner H, Falkner B (1999) Low dietary magnesium is associated with insulin resistance in a sample of young, nondiabetic Black Americans. Am J Hypertens 12:747–776
Huerta GM, Roemmich JN, Kington ML et al (2005) Magnesium deficiency is associated with insulin resistance in obese children. Diabetes Care 28:175–181
Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, Duh EJ, Vinores SA (2011) TNF alpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. IOVS 52(3):1336–1344
Joussen AM, Doehmen S, Le LM, Koizumi K, Radetzky S (2009) TNF-α mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol Vis 15:1418–1428
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jindal, V. Neurodegeneration as a Primary Change and Role of Neuroprotection in Diabetic Retinopathy. Mol Neurobiol 51, 878–884 (2015). https://doi.org/10.1007/s12035-014-8732-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8732-7