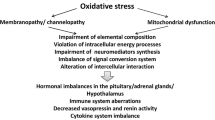
Abstract
Many patients with systemic immune-inflammatory and neuro-inflammatory disorders, including depression, rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s disease, cancer, cardiovascular disorder, Parkinson’s disease, multiple sclerosis, stroke, and chronic fatigue syndrome/myalgic encephalomyelitis, endure pathological levels of fatigue. The aim of this narrative review is to delineate the wide array of pathways that may underpin the incapacitating fatigue occurring in systemic and neuro-inflammatory disorders. A wide array of immune, inflammatory, oxidative and nitrosative stress (O&NS), bioenergetic, and neurophysiological abnormalities are involved in the etiopathology of these disease states and may underpin the incapacitating fatigue that accompanies these disorders. This range of abnormalities comprises: increased levels of pro-inflammatory cytokines, e.g., interleukin-1 (IL-1), IL-6, tumor necrosis factor (TNF) α and interferon (IFN) α; O&NS-induced muscle fatigue; activation of the Toll-Like Receptor Cycle through pathogen-associated (PAMPs) and damage-associated (DAMPs) molecular patterns, including heat shock proteins; altered glutaminergic and dopaminergic neurotransmission; mitochondrial dysfunctions; and O&NS-induced defects in the sodium-potassium pump. Fatigue is also associated with altered activities in specific brain regions and muscle pathology, such as reductions in maximum voluntary muscle force, downregulation of the mitochondrial biogenesis master gene peroxisome proliferator-activated receptor gamma coactivator 1-alpha, a shift to glycolysis and buildup of toxic metabolites within myocytes. As such, both mental and physical fatigue, which frequently accompany immune-inflammatory and neuro-inflammatory disorders, are the consequence of interactions between multiple systemic and central pathways.


Similar content being viewed by others
References
Weis J (2011) Cancer-related fatigue: prevalence, assessment and treatment strategies. Expert Rev Pharmacoecon Outcomes Res 11(4):441–6. doi:10.1586/erp.11.44
Segal B, Thomas W, Rogers T, Leon JM, Hughes P, Patel D, Patel K, Novitzke J, Rohrer M, Gopalakrishnan R, Myers S, Nazmul-Hossain A, Emamian E, Huang A, Rhodus N, Moser K (2008) Prevalence, severity, and predictors of fatigue in subjects with primary Sjögren's syndrome. Arthritis Rheum 59(12):1780–7. doi:10.1002/art.24311
Ahn GE, Ramsey-Goldman R (2012) Fatigue in systemic lupus erythematosus. Int J Clin Rheumatol 7(2):217–227
Hewlett S, Ambler N, Almeida C, Cliss A, Hammond A, Kitchen K, Knops B, Pope D, Spears M, Swinkels A, Pollock J (2011) Self-management of fatigue in rheumatoid arthritis: a randomised controlled trial of group cognitive-behavioural therapy. Ann Rheum Dis 70(6):1060–7. doi:10.1136/ard.2010.144691
Morris G, Maes M (2013) Myalgic encephalomyelitis/chronic fatigue syndrome and encephalomyelitis disseminata/multiple sclerosis show remarkable levels of similarity in phenomenology and neuroimmune characteristics. BMC Med 11:205. doi:10.1186/1741-7015-11-205
Beiske AG, Svensson E (2010) Fatigue in Parkinson's disease: a short update. Acta Neurol Scand Suppl 190:78–81. doi:10.1111/j.1600-0404.2010.01381.x
Winward C, Sackley C, Metha Z, Rothwell PM (2009) A population-based study of the prevalence of fatigue after transient ischemic attack and minor stroke. Stroke 40(3):757–61. doi:10.1161/STROKEAHA.108.527101
Alsén P, Brink E, Persson LO, Brändström Y, Karlson BW (2010) Illness perceptions after myocardial infarction: relations to fatigue, emotional distress, and health-related quality of life. J Cardiovasc Nurs 25(2):E1–E10. doi:10.1097/JCN.0b013e3181c6dcfd
Maes M, Kubera M, Obuchowiczwa E, Goehler L, Brzeszcz J (2011) Depression’s multiple comorbidities explained by (neuro) inflammatory and oxidative & nitrosative stress pathways. Neuro Endocrinol Lett 32(1):7–24
Thase ME (2009) Atypical depression: useful concept, but it’s time to revise the DSM-IV criteria. Neuropsychopharmacology 34(13):2633–41. doi:10.1038/npp.2009.100
Al-shair K, Muellerova H, Yorke J, Rennard SI, Wouters EF, Hanania NA, Sharafkhaneh A, Vestbo J; ECLIPSE investigators. Examining fatigue in COPD: development, validity and reliability of a modified version of FACIT-F scale. Health Qual Life Outcomes. 2012 23;10:100. doi: 10.1186/1477-7525-10-100. PubMed PMID: 22913289; PubMed Central PMCID: PMC3491053.
Norrie J, Heitger M, Leathem J, Anderson T, Jones R, Flett R (2010) Mild traumatic brain injury and fatigue: a prospective longitudinal study. Brain Inj 24(13–14):1528–38. doi:10.3109/02699052.2010.531687
Römkens TE, van Vugt-van Pinxteren MW, Nagengast FM, van Oijen MG, de Jong DJ (2011) High prevalence of fatigue in inflammatory bowel disease: a case control study. J Crohns Colitis 5(4):332–7. doi:10.1016/j.crohns.2011.02.008
Colosimo C, Millefiorini E, Grasso MG, Vinci F, Fiorelli M, Koudriavtseva T, Pozzilli C (1995) Fatigue in MS is associated with specific clinical features. Acta Neurol Scand 92(5):353–5
Krupp LB, Pollina DA (1996) Mechanisms and management of fatigue in progressive neurological disorders. Curr Opin Neurol 9(6):456–60
Ford H, Trigwell P, Johnson M (1998) The nature of fatigue in multiple sclerosis. J Psychosom Res 45(1):33–8
Schreurs KM, de Ridder DT, Bensing JM (2002) Fatigue in multiple sclerosis: reciprocal relationships with physical disabilities and depression. J Psychosom Res 53(3):775–81
Flachenecker P, Bihler I, Weber F, Gottschalk M, Toyka KV, Rieckmann P (2004) Cytokine mRNA expression in patients with multiple sclerosis and fatigue. Mult Scler 10(2):165–9
Chaudhuri A, Behan PO (2000) Fatigue and basal ganglia. J Neurol Sci 179(S 1–2):34–42
Lindqvist G, Malmgren H (1993) Organic mental disorders as hypothetical pathogenetic processes. Acta Psychiatr Scand Suppl 373:5–17
Morris G, Maes M (2014) Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab Brain Dis 29(1):19–36. doi:10.1007/s11011-013-9435-x
Blach-Olszewska Z, Leszek J (2007) Mechanisms of over-activated innate immune system regulation in autoimmune and neurodegenerative disorders. Neuropsychiatr Dis Treat 3(3):365–72
Perl A (2013) Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat Rev Rheumatol 9(11):674–86. doi:10.1038/nrrheum.2013.147
Pagano G, Castello G, Pallardó FV (2013) Sjøgren’s syndrome-associated oxidative stress and mitochondrial dysfunction: prospects for chemoprevention trials. Free Radic Res 47(2):71–3. doi:10.3109/10715762.2012.748904
Szabó-Taylor KE, Nagy G, Eggleton P, Winyard PG. 2013. Oxidative stress in rheumatoid arthritis. In: Studies on arthritis and joint disorders. pp.145–167. DOI 10.1007/978-1-4614-6166-1_8. Springer New York
Exner N, Lutz AK, Haass C, Winklhofer KF (2012) Mitochondrial dysfunction in Parkinson’s disease: molecular mechanisms and pathophysiological consequences. EMBO J 31(14):3038–62. doi:10.1038/emboj.2012.170
Allen CL, Bayraktutan U (2009) Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke 4(6):461–70. doi:10.1111/j.1747-4949.2009.00387.x
Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, LLeonart ME (2013) Oxidative stress and cancer: an overview. Ageing Res Rev 12(1):376–90. doi:10.1016/j.arr.2012.10.004
Moylan S, Berk M, Dean OM, Samuni Y, Williams LJ, O’Neil A, Hayley AC, Pasco JA, Anderson G, Jacka FN, Maes M (2014) Oxidative & nitrosative stress in depression: why so much stress? Neurosci Biobehav Rev 45C:46–62. doi:10.1016/j.neubiorev.2014.05.007
Pfaffenseller B, Fries GR, Wollenhaupt-Aguiar B, Colpo GD, Stertz L, Panizzutti B, Magalhães PV, Kapczinski F (2013) Neurotrophins, inflammation and oxidative stress as illness activity biomarkers in bipolar disorder. Expert Rev Neurother 13(7):827–42. doi:10.1586/14737175.2013.811981
Pieczenik SR, Neustadt J (2007) Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol 83(1):84–92
Hwang O (2013) Role of oxidative stress in Parkinson’s disease. Exp Neurobiol 22:11–17. doi:10.5607/en.2013.22.1.11
Scalzo P, Kümmer A, Cardoso F, Teixeira AL (2009) Increased serum levels of soluble tumor necrosis factor-alpha receptor-1 in patients with Parkinson’s disease. J Neuroimmunol 216(1–2):122–5. doi:10.1016/j.jneuroim.2009.08.001
Heesen C, Nawrath L, Reich C, Bauer N, Schulz KH, Gold SM (2006) Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? J Neurol Neurosurg Psychiatry 77(1):34–9
Menzies V, Lyon DE (2010) Integrated review of the association of cytokines with fibromyalgia and fibromyalgia core symptoms. Biol Res Nurs 11(4):387–94. doi:10.1177/1099800409348328
Seruga B, Zhang H, Bernstein LJ, Tannock IF (2008) Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer 8(11):887–99. doi:10.1038/nrc2507
Patarca R (2001) Cytokines and chronic fatigue syndrome. Ann N Y Acad Sci 933:185–200
Nakamura T, Schwander SK, Donnelly R, Ortega F, Togo F, Broderick G, Yamamoto Y, Cherniack NS, Rapoport D, Natelson BH (2010) Cytokines across the night in chronic fatigue syndrome with and without fibromyalgia. Clin Vaccine Immunol 17(4):582–7. doi:10.1128/CVI.00379-09
Maes M (1993) A review on the acute phase response in major depression. Rev Neurosci 4(4):407–16
Tyrrell PJ, Smithard DG (2006) Fatigue after stroke. Therapy 2:865–869. doi:10.2217/14750708.2.6.865
Emsley HC, Tyrrell PJ (2002) Inflammation and infection in clinical stroke. J Cereb Blood Flow Metab 22(12):1399–419
Ormstad H, Aass HC, Amthor KF, Lund-Sørensen N, Sandvik L. Serum cytokine and glucose levels as predictors of poststroke fatigue in acute ischemic stroke patients. J Neurol. 2011 Apr;258(4):670–6. doi: 10.1007/s00415-011-5962-8. Epub 2011 Mar 2. Erratum in: J Neurol. 2012 Feb;259(2):399. PubMed PMID: 21365457; PubMed Central PMCID: PMC3065647
Yirmiya R (2000) Depression in medical illness: the role of the immune system. West J Med 173(5):333–6
Yirmiya R, Weidenfeld J, Pollak Y, Morag M, Morag A, Avitsur R, Barak O, Reichenberg A, Cohen E, Shavit Y, Ovadia H (1999) Cytokines, “depression due to a general medical condition,” and antidepressant drugs. Adv Exp Med Biol 461:283–316
Maes M, Kubera M, Leunis JC, Berk M (2012) Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J Affect Disord 141(1):55–62. doi:10.1016/j.jad.2012.02.023
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55(5):453–62
Burton MD, Sparkman NL, Johnson RW (2011) Inhibition of interleukin-6 trans-signaling in the brain facilitates recovery from lipopolysaccharide-induced sickness behavior. J Neuroinflammation 8:54. doi:10.1186/1742-2094-8-54
Kluger MJ (1980) Fever Pediatrics 66(5):720–4
Leonard BE, Song C (2002) Changes in the immune system in rodent models of depression. Int J Neuropsychopharmacol 5(4):345–56
Song C, Leonard BE (2005) The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev 29(4–5):627–47
Goehler LE, Lyte M, Gaykema RP (2007) Infection-induced viscerosensory signals from the gut enhance anxiety: implications for psychoneuroimmunology. Brain Behav Immun 21(6):721–6
Anisman H, Merali Z (1999) Anhedonic and anxiogenic effects of cytokine exposure. Adv Exp Med Biol 461:199–233
Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE (2006) Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav 89(3):350–7
Gaykema RP, Goehler LE (2009) Lipopolysaccharide challenge-induced suppression of Fos in hypothalamic orexin neurons: their potential role in sickness behavior. Brain Behav Immun 23(7):926–30. doi:10.1016/j.bbi.2009.03.005
Morris G, Anderson G, Galecki P, Berk M, Maes M (2013) A narrative review on the similarities and dissimilarities between myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and sickness behavior. BMC Med 11:64. doi:10.1186/1741-7015-11-64
Morris G, Maes M (2013) A neuro-immune model of myalgic encephalomyelitis/chronic fatigue syndrome. Metab Brain Dis 28(4):523–40. doi:10.1007/s11011-012-9324-8
Peters A (2006) The energy request of inflammation. Endocrinology 147(10):4550–2
Schaffner A (2006) [Fever—useful or noxious symptom that should be treated?]. Ther Umsch 63(3):185–8
Rantala S, Vuopio-Varkila J, Vuento R, Huhtala H, Syrjänen J (2009) Predictors of mortality in beta-hemolytic streptococcal bacteremia: a population-based study. J Infect 58(4):266–72. doi:10.1016/j.jinf.2009.01.015
Charlton BG (2000) The malaise theory of depression: major depressive disorder is sickness behavior and antidepressants are analgesic. Med Hypotheses 54(1):126–30
Felger JC, Li L, Marvar PJ, Woolwine BJ, Harrison DG, Raison CL, Miller AH (2013) Tyrosine metabolism during interferon-alpha administration: association with fatigue and CSF dopamine concentrations. Brain Behav Immun 31:153–60. doi:10.1016/j.bbi.2012.10.010
Kirkwood J (2002) Cancer immunotherapy: the interferon-alpha experience. Semin Oncol 29(3 Suppl 7):18–26
Dorr RT (1993) Interferon-alpha in malignant and viral diseases. A review Drugs 45(2):177–211
Ohga S, Aoki T, Okada K, Akeda H, Fujioka K, Ohshima A, Mori T, Minamishima I, Ueda K (1994) Cerebrospinal fluid concentrations of interleukin-1 beta, tumour necrosis factor-alpha, and interferon gamma in bacterial meningitis. Arch Dis Child 70(2):123–5
Barth PG. The neuropathology of Aicardi-Goutières syndrome. Eur J Paediatr Neurol. 2002;6 Suppl A:A27-31; discussion A37-9, A77-86. PubMed PMID: 12365358.
Jönsen A, Bengtsson AA, Nived O, Ryberg B, Truedsson L, Rönnblom L, Alm GV, Sturfelt G (2003) The heterogeneity of neuropsychiatric systemic lupus erythematosus is reflected in lack of association with cerebrospinal fluid cytokine profiles. Lupus 12(11):846–50
Wichers MC, Koek GH, Robaeys G et al (2005) Early increase in vegetative symptoms predicts IFN-alpha-induced cognitive-depressive changes. Psy- chol Med 35:433–441
Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH (2008) IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun 22(6):870–80. doi:10.1016/j.bbi.2007.12.009
Sunami M, Nishikawa T, Yorogi A, Shimoda M (2000) Intravenous administration of levodopa ameliorated a refractory akathisia case induced by interferon-alpha. Clin Neuropharmacol 23(1):59–61
Morrow GR, Hickok JT, Roscoe JA, Raubertas RF, Andrews PL, Flynn PJ, Hynes HE, Banerjee TK, Kirshner JJ, King DK (2003) University of Rochester Cancer Center Community Clinical Oncology Program. Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol 21(24):4635–41
Raison CL, Demetrashvili M, Capuron L, Miller AH (2005) Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs 19(2):105–23
Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, Whedon MB, Bivens S, Mitchell T, Greenberg ER, Silberfarb PM (2002) Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol 20(2):485–93
Bower JE, Ganz PA, Aziz N, Fahey JL (2002) Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med 64(4):604–11
Rönnbäck L, Hansson E (2004) On the potential role of glutamate transport in mental fatigue. J Neuroinflammation 1(1):22
Minagar A, Alexander JS (2003) Blood-brain barrier disruption in multiple sclerosis. Mult Scler 9(6):540–9
Lynch G (2004) AMPA receptor modulators as cognitive enhancers. Curr Opin Pharmacol 4(1):4–11
Sibson NR, Blamire AM, Bernades-Silva M, Laurent S, Boutry S, Muller RN, Styles P, Anthony DC. MRI detection of early endothelial activation in brain inflammation. Magn Reson Med. 2004 Feb;51(2):248–52. PubMed PMID: 14755648. 2004 Feb;4(1):4–11. Review. PubMed PMID: 15018832.
Morris G, Berk M, Galecki P, Maes M (2014) The emerging role of autoimmunity in myalgic encephalomyelitis/chronic fatigue syndrome (ME/cfs). Mol Neurobiol 49(2):741–56. doi:10.1007/s12035-013-8553-0
Lopez-Ramirez MA, Male DK, Wang C, Sharrack B, Wu D, Romero IA (2013) Cytokine-induced changes in the gene expression profile of a human cerebral microvascular endothelial cell-line, hCMEC/D3. Fluids Barriers CNS 10(1):27. doi:10.1186/2045-8118-10-27
Haroon E, Anand, R.; Chen, X.; Ford, R.; Parekh, S.; Woolwine, B.; Spivey, J.R.; Hu, X.; Miller, A.H. 2012. 178. Interferon-alpha-induced fatigue is associated with alterations in CNS glutamate metabolism as measured by magnetic resonance spectroscopy. Brain Behavior and Immunity vol. 26 September, Supple 1 p. S49–S50.
Yirmiya R, Goshen I (2011) Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 25(2):181–213. doi:10.1016/j.bbi.2010.10.015
Collins JM, Riccardi R, Trown P, O’Neill D, Poplack DG (1985) Plasma and cerebrospinal fluid pharmacokinetics of recombinant interferon alpha A in monkeys: comparison of intravenous, intramuscular, and intraventricular delivery. Cancer Drug Deliv 2(4):247–53
Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, Kalin NH, Ratti E, Nemeroff CB, Miller AH (2007) Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol Psychiatry 62(11):1324–33
Smith RA, Norris F, Palmer D, Bernhardt L, Wills RJ (1985) Distribution of alpha interferon in serum and cerebrospinal fluid after systemic administration. Clin Pharmacol Ther 37(1):85–8
Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV (2005) Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab 25(5):593–606
Weiss JM, Downie SA, Lyman WD, Berman JW (1998) Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood-brain barrier. J Immunol 161(12):6896–903
Hokeness KL, Kuziel WA, Biron CA, Salazar-Mather TP (2005) Monocyte chemoattractant protein-1 and CCR2 interactions are required for IFN-alpha/beta-induced inflammatory responses and antiviral defense in liver. J Immunol 174(3):1549–56
Rankine EL, Hughes PM, Botham MS, Perry VH, Felton LM (2006) Brain cytokine synthesis induced by an intraparenchymal injection of LPS is reduced in MCP-1-deficient mice prior to leucocyte recruitment. Eur J Neurosci 24(1):77–86
Akiyama H, Ikeda K, Katoh M, McGeer EG, McGeer PL (1994) Expression of MRP14, 27E10, interferon-alpha and leukocyte common antigen by reactive microglia in postmortem human brain tissue. J Neuroimmunol 50(2):195–201
Curtin JF, King GD, Barcia C, Liu C, Hubert FX, Guillonneau C, Josien R, Anegon I, Lowenstein PR, Castro MG (2006) Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol 176(6):3566–77
Colton CA, Yao J, Keri JE, Gilbert D (1992) Regulation of microglial function by interferons. J Neuroimmunol 40(1):89–98
Felger JC, Miller AH (2012) Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol 33(3):315–27. doi:10.1016/j.yfrne.2012.09.003
Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH (2010) CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry 15(4):393–403. doi:10.1038/mp.2009.116
Indraccolo S, Pfeffer U, Minuzzo S, Esposito G, Roni V, Mandruzzato S, Ferrari N, Anfosso L, Dell’Eva R, Noonan DM, Chieco-Bianchi L, Albini A, Amadori A (2007) Identification of genes selectively regulated by IFNs in endothelial cells. J Immunol 178(2):1122–35
Tissari J, Sirén J, Meri S, Julkunen I, Matikainen S (2005) IFN-alpha enhances TLR3-mediated antiviral cytokine expression in human endothelial and epithelial cells by up-regulating TLR3 expression. J Immunol 174(7):4289–94
Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T (2005) IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434(7034):772–7
Wang J, Campbell IL, Zhang H (2008) Systemic interferon-alpha regulates interferon-stimulated genes in the central nervous system. Mol Psychiatry 13(3):293–301
Pan W, Banks WA, Kastin AJ (1997) Permeability of the blood-brain and blood-spinal cord barriers to interferons. J Neuroimmunol 76(1–2):105–11
Nicolson GL, Settineri R (2011) Lipid replacement therapy: a functional food approach with new. Formulations for reducing cellular oxidative damage. Cancer-Associated Functional foods in health and disease 1(4):135–160
Segal BM, Thomas W, Zhu X, Diebes A, McElvain G, Baechler E, Gross M (2012) Oxidative stress and fatigue in systemic lupus erythematosus. Lupus 21(9):984–92. doi:10.1177/0961203312444772
Cordero MD, Alcocer-Gómez E, de Miguel M, Cano-García FJ, Luque CM, Fernández-Riejo P, Fernández AM, Sánchez-Alcazar JA (2011) Coenzyme Q(10): a novel therapeutic approach for fibromyalgia? Case series with 5 patients. Mitochondrion 11(4):623–5. doi:10.1016/j.mito.2011.03.122
Avalos I, Chung CP, Oeser A, Milne GL, Morrow JD, Gebretsadik T, Shintani A, Yu C, Stein CM (2007) Oxidative stress in systemic lupus erythematosus: relationship to disease activity and symptoms. Lupus 16(3):195–200
Kennedy G, Spence VA, McLaren M, Hill A, Underwood C, Belch JJ (2005) Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radic Biol Med 39(5):584–9
Jammes Y, Steinberg JG, Delliaux S, Brégeon F (2009) Chronic fatigue syndrome combines increased exercise-induced oxidative stress and reduced cytokine and Hsp responses. J Intern Med 266(2):196–206. doi:10.1111/j.1365-2796.2009.02079.x
Jammes Y, Steinberg JG, Delliaux S (2012) Chronic fatigue syndrome: acute infection and history of physical activity affect resting levels and response to exercise of plasma oxidant/antioxidant status and heat shock proteins. J Intern Med 272(1):74–84. doi:10.1111/j.1365-2796.2011.02488.x
Rodrigo R, Fernandez-Gajardo R, Gutierrez R et al (2013) Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS Neurol Disord Drug Targets 12(5):698–714
Chen XM, Chen HS, Xu MJ, Shen JG (2013) Targeting reactive nitrogen species: a promising therapeutic strategy for cerebral ischemia-reperfusion injury. Acta Pharmacol Sin 34(1):67–77. doi:10.1038/aps.2012.82
Wang G, Pierangeli SS, Papalardo E, Ansari GA, Khan MF (2010) Markers of oxidative and nitrosative stress in systemic lupus erythematosus: correlation with disease activity. Arthritis Rheum 62(7):2064–72. doi:10.1002/art.27442
Wakamatsu TH, Dogru M, Matsumoto Y, Kojima T, Kaido M, Ibrahim OM, Sato EA, Igarashi A, Ichihashi Y, Satake Y, Shimazaki J, Tsubota K (2013) Evaluation of lipid oxidative stress status in Sjögren syndrome patients. Invest Ophthalmol Vis Sci 54(1):201–10. doi:10.1167/iovs.12-10325
Karatas F, Ozates I, Canatan H, Halifeoglu I, Karatepe M, Colakt R (2003) Antioxidant status & lipid peroxidation in patients with rheumatoid arthritis. Indian J Med Res 118:178–81
Chandankhede MS, Gupta MM (2013) Oxidative stress and antioxidant status in patients with rheumatoid arthritis. Int J Biol Med Res 4(2):3088–3090
Farooqui T, Farooqui AA (2011) Lipid-mediated oxidative stress and inflammation in the pathogenesis of Parkinson’s disease. Parkinsons Dis 2011:247467
O’Neill CA, Stebbins CL, Bonigut S, Halliwell B, Longhurst JC (1996) Production of hydroxyl radicals in contracting skeletal muscle of cats. J Appl Physiol 81(3):1197–1206
Pattwell DM, McArdle A, Morgan JE, Patridge TA, Jackson MJ (2004) Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic Biol Med 37(7):1064–1072
Ferreira LF, Reid MB (2008) Muscle-derived ROS and thiol regulation in muscle fatigue. J Appl Physiol 104(3):853–860
Jackson MJ, Pye D, Palomero J (2007) The production of reactive oxygen and nitrogen species by skeletal muscle. J Appl Physiol 102(4):1664–1670
Albertini M, Lafortuna C, Aguggini G (1997) Effects of nitric oxide on diaphragmatic muscle endurance and strength in pigs. Exp Physiol 82(1):99–106
Belia S, Pietrangelo T, Fulle S, Menchetti G, Cecchini E, Felaco M, Vecchiet J, Fanò G (1998) Sodium nitroprusside, a NO donor, modifies Ca2+ transport and mechanical properties in frog skeletal muscle. J Muscle Res Cell Motil 19(8):865–76
Zhu X, Heunks LM, Versteeg EM, van der Heijden HF, Ennen L, van Kuppevelt TH, Vina J, Dekhuijzen PN (2005) Hypoxia-induced dysfunction of rat diaphragm: role of peroxynitrite. Am J Physiol Lung Cell Mol Physiol 288(1):L16–26
Boczkowski J, Lanone S, Ungureanu-Longrois D, Danialou G, Fournier T, Aubier M (1996) Induction of diaphragmatic nitric oxide synthase after endotoxin administration in rats: role on diaphragmatic contractile dysfunction. J Clin Invest 98(7):1550–1559
Hambrecht R, Adams V, Gielen S, Linke A, Möbius-Winkler S, Yu J, Niebauer J, Jiang H, Fiehn E, Schuler G (1999) Exercise intolerance in patients with chronic heart failure and increased expression of inducible nitric oxide synthase in the skeletal muscle. J Am Coll Cardiol 33(1):174–179
Rubinstein I, Abassi Z, Coleman R, Milman F, Winaver J, Better OS (1998) Involvement of nitric oxide system in experimental muscle crush injury. J Clin Invest 101(6):1325–1333
Reid MB, Stokić DS, Koch SM, Khawli FA, Leis AA (1994) N-acetylcysteine inhibits muscle fatigue in humans. J Clin Invest 94(6):2468–2474
Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X, McKenna MJ (2004) N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol 97:1477–1485
Reid MB (2001) Invited review: redox modulation of skeletal muscle contraction: what we know and what we don’t. J Appl Physiol 90(2):724–731
Smith MA, Reid MB (2006) Redox modulation of contractile function in respiratory and limb skeletal muscle. Respir Physiol Neurobiol 151(2–3):229–241
Fujii Y, Takahashi S, Toyooka H (2013) Protection from diaphragmatic fatigue by nitric oxide synthase inhibitor in dogs. Anaesth Intensive Care. 1999 Feb;27(1):45–8. Retraction in: Gibbs N. Anaesth Intensive Care 41(2):275
Grassi B, Hogan MC, Kelley KM, Howlett RA, Gladden LB (2005) Effects of nitric oxide synthase inhibition by l-NAME on oxygen uptake kinetics in isolated canine muscle in situ. J Physiol 568(Pt 3):1021–33
Hart JD, Dulhunty AF (2000) Nitric oxide activates or inhibits skeletal muscle ryanodine receptors depending on its concentration, membrane potential and ligand binding. J Membr Biol 173(3):227–36
Ishii T, Sunami O, Saitoh N, Nishio H, Takeuchi T, Hata F (1998) Inhibition of skeletal muscle sarcoplasmic reticulum Ca2+-ATPase by nitric oxide. FEBS Lett 440(1–2):218–22
Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH (1994) Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases FEBS Lett 345(1):50–4
Davies KJ, Quintanilha AT, Brooks GA, Packer L (1982) Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun 107(4):1198–205
Pouvreau S, Allard B, Berthier C, Jacquemond V (2004) Control of intracellular calcium in the presence of nitric oxide donors in isolated skeletal muscle fibres from mouse. J Physiol 560(Pt 3):779–794
Kobzik L, Reid MB, Bredt DS, Stamler JS (1994) Nitric oxide in skeletal muscle. Nature 372(6506):546–548
Thaloor D, Miller KJ, Gephart J, Mitchell PO, Pavlath GK (1999) Systemic administration of the NF-kappaB inhibitor curcumin stimulates muscle regeneration after traumatic injury. Am J Physiol 277(2 Pt 1):C320–C329
Mourkioti F, Kratsios P, Luedde T, Song YH, Delafontaine P, Adami R, Parente V, Bottinelli R, Pasparakis M, Rosenthal N (2006) Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J Clin Invest 116(11):2945–2954
Bar-Shai M, Carmeli E, Reznick AZ (2005) The role of NF-kappaB in protein breakdown in immobilization, aging, and exercise: from basic processes to promotion of health. Ann N Y Acad Sci 1057:431–47
Buck M, Chojkier M (1996) Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J 15(8):1753–1765
Barbieri E, Sestili P. Reactive oxygen species in skeletal muscle signaling. J Signal Transduct. 2012;2012:982794. doi: 10.1155/2012/982794. Epub 2011 Dec 5. PubMed PMID: 22175016; PubMed Central PMCID: PMC3235811
Forcales SV, Puri PL (2005) Signaling to the chromatin during skeletal myogenesis: novel targets for pharmacological modulation of gene expression. Semin Cell Dev Biol 16(4–5):596–611
Messina S, Altavilla D, Aguennouz M, Seminara P, Minutoli L, Monici MC, Bitto A, Mazzeo A, Marini H, Squadrito F, Vita G (2006) Lipid peroxidation inhibition blunts nuclear factor-kappaB activation, reduces skeletal muscle degeneration, and enhances muscle function in mdx mice. Am J Pathol 168(3):918–26
Fulle S, Protasi F, Di Tano G, Pietrangelo T, Beltramin A, Boncompagni S, Vecchiet L, Fanò G (2004) The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp Gerontol 39(1):17–24
Lucas K, Maes M (2013) Role of the Toll Like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol Neurobiol 48(1):190–204. doi:10.1007/s12035-013-8425-7
Guijarro-Muñoz I, Compte M, Álvarez-Cienfuegos A, Álvarez-Vallina L, Sanz L (2014) Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-κB signaling pathway and proinflammatory response in human pericytes. J Biol Chem 289(4):2457–68. doi:10.1074/jbc.M113.521161
Yang Y, Kim SC, Yu T, Yi YS, Rhee MH, Sung GH, Yoo BC, Cho JY (2014) Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators Inflamm 2014:352371. doi:10.1155/2014/352371
Weismann D, Binder CJ (2012) The innate immune response to products of phospholipid peroxidation. Biochim Biophys Acta 1818(10):2465–75. doi:10.1016/j.bbamem.2012.01.018
Ellerman JE, Brown CK, de Vera M, Zeh HJ, Billiar T, Rubartelli A, Lotze MT (2007) Masquerader: high mobility group box-1 and cancer. Clin Cancer Res 13(10):2836–48
Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A (2008) HMGB1: endogenous danger signaling. Mol Med 14(7–8):476–84
Srikrishna G, Freeze HH (2009) Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia 11(7):615–28
Sato Y, Goto Y, Narita N, Hoon DS (2009) Cancer cells expressing toll-like receptors and the tumor microenvironment. Cancer Microenviron 2(Suppl 1):205–14
Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J (2007) Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 13(9):1042–9
Uchida K (2013) Redox-derived damage-associated molecular patterns: ligand function of lipid peroxidation adducts. Redox Biol 1(1):94–6. doi:10.1016/j.redox.2012.12.005
Carta S, Castellani P, Delfino L, Tassi S, Venè R, Rubartelli A (2009) DAMPs and inflammatory processes: the role of redox in the different outcomes. J Leukoc Biol 86(3):549–55. doi:10.1189/jlb.1008598
Moghaddam AE, Gartlan KH, Kong L, Sattentau QJ (2011) Reactive carbonyls are a major Th2-inducing damage-associated molecular pattern generated by oxidative stress. J Immunol 187(4):1626–33
Bruenner BA, Jones AD, German JB (1995) Direct characterization of protein adducts of the lipid peroxidation product 4-hydroxy-2-nonenal using electrospray mass spectrometry. Chem Res Toxicol 8:552–559
Ishii T, Kumazawa S, Sakurai T, Nakayama T, Uchida K (2006) Mass spectroscopic characterization of protein modification by malondialdehyde. Chem Res Toxicol 19(1):122–9
Simmons JD, Lee YL, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, Gillespie MN, Richards WO (2013) Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg 258(4):591–6. doi:10.1097/SLA.0b013e3182a4ea46
Mathew A, Lindsley TA, Sheridan A, Bhoiwala DL, Hushmendy SF, Yager EJ, Ruggiero EA, Crawford DR (2012) Degraded mitochondrial DNA is a newly identified subtype of the damage associated molecular pattern (DAMP) family and possible trigger of neurodegeneration. J Alzheimers Dis 30(3):617–27
Hsu GJ, Tzang BS, Tsai CC, Chiu CC, Huang CY, Hsu TC (2011) Effects of human parvovirus B19 on expression of defensins and Toll-like receptors. Chin J Physiol 54(5):367–76
Frisancho-Kiss S, Davis SE, Nyland JF et al (2007) Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol 178:6710–6714
Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, Finberg RW (2003) Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol 77(8):4588–96
Zamboni DS, Campos MA, Torrecilhas AC, Kiss K, Samuel JE, Golenbock DT, Lauw FN, Roy CR, Almeida IC, Gazzinelli RT (2004) Stimulation of toll-like receptor 2 by Coxiella burnetii is required for macrophage production of pro-inflammatory cytokines and resistance to infection. J Biol Chem 279(52):54405–15
Salazar JC, Duhnam-Ems S, La Vake C, Cruz AR, Moore MW, Caimano MJ, Velez-Climent L, Shupe J, Krueger W, Radolf JD (2009) Activation of human monocytes by live Borrelia burgdorferi generates TLR2-dependent and -independent responses which include induction of IFN-beta. PLoS Pathog 5(5):e1000444
Friis LM, Keelan M, Taylor DE (2009) Campylobacter jejuni drives MyD88-independent interleukin-6 secretion via Toll-like receptor 2. Infect Immun 77(4):1553–60
Gow JW, Hagan S, Herzyk P, Cannon C, Behan PO, Chaudhuri A (2009) A gene signature for post-infectious chronic fatigue syndrome. BMC Med Genomics 2:38. doi:10.1186/1755-8794-2-38
Light AR, Vierck CJ, Light KC (2010) Myalgia and Fatigue: Translation from Mouse Sensory Neurons to Fibromyalgia and Chronic Fatigue Syndromes. In: Kruger L, Light AR (eds) Translational pain research: from mouse to man. CRC, Boca Raton, FL
Nagyoszi P, Wilhelm I, Farkas AE, Fazakas C, Dung NT, Haskó J, Krizbai IA (2010) Expression and regulation of toll-like receptors in cerebral endothelial cells. Neurochem Int 57(5):556–64
Andersson A, Covacu R, Sunnemark D, Danilov AI, Dal Bianco A, Khademi M, Wallström E, Lobell A, Brundin L, Lassmann H, Harris RA (2008) Pivotal advance: HMGB1 expression in active lesions of human and experimental multiple sclerosis. J Leukoc Biol 84(5):1248–55
Bsibsi M, Ravid R, Gveric D, van Noort JM (2002) Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol 61(11):1013–21
Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, Joong Lee S, Masliah E, Hwang D, Lee HJ, Lee SJ (2013) Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun 4:1562
Pandey GN, Rizavi HS, Ren X, Bhaumik R, Dwivedi Y (2014) Toll-like receptors in the depressed and suicide brain. J Psychiatr Res 53:62–8. doi:10.1016/j.jpsychires.2014.01.021
Tiffin N, Adeyemo A, Okpechi I (2013) A diverse array of genetic factors contribute to the pathogenesis of systemic lupus erythematosus. Orphanet J Rare Dis 8:2. doi:10.1186/1750-1172-8-2
Low HZ, Witte T (2011) Aspects of innate immunity in Sjögren’s syndrome. Arthritis Res Ther 13(3):218. doi:10.1186/ar3318
Goh FG, Midwood KS (2012) Intrinsic danger: activation of Toll-like receptors in rheumatoid arthritis. Rheumatology (Oxford) 51(1):7–23. doi:10.1093/rheumatology/ker257
Wang PF, Fang H, Chen J, Lin S, Liu Y, Xiong XY, Wang YC, Xiong RP, Lv FL, Wang J, Yang QW (2014) Polyinosinic-polycytidylic acid has therapeutic effects against cerebral ischemia/reperfusion injury through the downregulation of TLR4 signaling via TLR3. J Immunol 192(10):4783–94. doi:10.4049/jimmunol.1303108
Vereker E, O’Donnell E, Lynch MA (2000) The inhibitory effect of interleukin-1beta on long-term potentiation is coupled with increased activity of stress-activated protein kinases. J Neurosci 20(18):6811–9
Yudkoff M, Nissim I, Daikhin Y, Lin ZP, Nelson D, Pleasure D, Erecinska M (1993) Brain glutamate metabolism: neuronal-astroglial relationships. Dev Neurosci 15(3–5):343–50
Huang YH, Bergles DE (2004) Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol 14(3):346–52
Pannasch U, Derangeon M, Chever O, Rouach N (2012) Astroglial gap junctions shape neuronal network activity. Communicative & integrative biology 5(3):248–254
Araque A (2008) Astrocytes process synaptic information. Neuron Glia Biol 4(01):3–10
Newman LA, Korol DL, Gold PE (2011) Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS One 6(12):e28427. doi:10.1371/journal.pone.0028427
Harrington, M. 2011. Astrocytes ‘feed’ memory formation. Lab animal, 40 (4), p. 98.
Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, Rothstein JD, Volsky DJ (2003) Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology 312(1):60–73
Fine SM, Angel RA, Perry SW, Epstein LG, Rothstein JD, Dewhurst S, Gelbard HA (1996) Tumor necrosis factor alpha inhibits glutamate uptake by primary human astrocytes. Implications for pathogenesis of HIV-1 dementia. J Biol Chem 271(26):15303–6
Hu S, Sheng WS, Ehrlich LC, Peterson PK, Chao CC (2000) Cytokine effects on glutamate uptake by human astrocytes. Neuroimmunomodulation 7(3):153–9
Chao CC, Hu S, Ehrlich L, Peterson PK (1995) Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-d-aspartate receptors. Brain Behav Immun 9(4):355–65
Loaiza A, Porras OH, Barros LF (2003) Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J Neurosci 23(19):7337–42
Hertz L. Intercellular metabolic compartmentation in the brain: past, present and future. Neurochem Int. 2004 Jul-Aug;45(2–3):285–96. Review. PubMed PMID: 15145544.
Waubant E (2006) Biomarkers indicative of blood-brain barrier disruption in multiple sclerosis. Dis Markers 22(4):235–244
Helms G (2001) Volume correction for edema in single-volume proton MR spectroscopy of contrast-enhancing multiple sclerosis lesions. Magn Reson Med 46(2):256–263
O’kane, R. L., Martínez-López, I., Dejoseph, M. R., Viña, J. R. and Hawkins, R. A. 1999. Na+-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood-brain Barrier A MECHANISM FOR GLUTAMATE REMOVAL. Journal of Biological Chemistry, 274 (45), pp. 31891–31895
Karpuk N, Burkovetskaya M, Fritz T, Angle A, Kielian T (2011) Neuroinflammation leads to region-dependent alterations in astrocyte gap junction communication and hemichannel activity. J Neurosci 31(2):414–25. doi:10.1523/JNEUROSCI.5247-10.2011
Takaki J, Fujimori K, Miura M, Suzuki T, Sekino Y, Sato K (2012) l-glutamate released from activated microglia downregulates astrocytic l-glutamate transporter expression in neuroinflammation: the ‘collusion’ hypothesis for increased extracellular l-glutamate concentration in neuroinflammation. J Neuroinflammation 9:275. doi:10.1186/1742-2094-9-275
Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, Seishima M (2006) The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem 139(4):655–62
Guillemin GJ, Smith DG, Smythe GA, Armati PJ, Brew BJ (2003) Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv Exp Med Biol 527:105–12
Pemberton LA, Kerr SJ, Smythe G, Brew BJ (1997) Quinolinic acid production by macrophages stimulated with IFN-gamma, TNF-alpha, and IFN-alpha. J Interferon Cytokine Res 17(10):589–95
Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR (1991) Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem 56(6):2007–17
Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ (2012) Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 13(7):465–77. doi:10.1038/nrn3257
Schwarcz R, Pellicciari R (2002) Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther 303(1):1–10
Tavares RG, Schmidt AP, Abud J, Tasca CI, Souza DO (2005) In vivo quinolinic acid increases synaptosomal glutamate release in rats: reversal by guanosine. Neurochem Res 30(4):439–44
Guidetti P, Schwarcz R (2003) 3-Hydroxykynurenine and quinolinate: pathogenic synergism in early grade Huntington’s disease? Adv Exp Med Biol 527:137–45
Guillemin GJ, Wang L, Brew BJ (2005) Quinolinic acid selectively induces apoptosis of human astrocytes: potential role in AIDS dementia complex. J Neuroinflammation 2:16
Stone TW (2000) Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends Pharmacol Sci 21(4):149–54
Ida T, Hara M, Nakamura Y, Kozaki S, Tsunoda S, Ihara H (2008) Cytokine-induced enhancement of calcium-dependent glutamate release from astrocytes mediated by nitric oxide. Neurosci Lett 432(3):232–6. doi:10.1016/j.neulet.2007.12.047
Tilleux S, Hermans E (2007) Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res 85(10):2059–70
Hardingham GE, Fukunaga Y, Bading H (2002) Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci 5(5):405–14
Haydon PG, Carmignoto G (2006) Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86(3):1009–31
Esposito Z, Belli L, Toniolo S, Sancesario G, Bianconi C, Martorana A (2013) Amyloid β, glutamate, excitotoxicity in Alzheimer’s disease: are we on the right track? CNS Neurosci Ther 19(8):549–55. doi:10.1111/cns.12095
Zinger A, Barcia C, Herrero MT, Guillemin GJ (2011) The involvement of neuroinflammation and kynurenine pathway in Parkinson’s disease. Parkinsons Dis 2011:716859. doi:10.4061/2011/716859
Vaarmann A, Kovac S, Holmström KM, Gandhi S, Abramov AY (2013) Dopamine protects neurons against glutamate-induced excitotoxicity. Cell Death Dis 4:e455. doi:10.1038/cddis.2012.194
Anitha A, Nakamura K, Thanseem I, Yamada K, Iwayama Y, Toyota T, Matsuzaki H, Miyachi T, Yamada S, Tsujii M, Tsuchiya KJ, Matsumoto K, Iwata Y, Suzuki K, Ichikawa H, Sugiyama T, Yoshikawa T, Mori N (2012) Brain region-specific altered expression and association of mitochondria-related genes in autism. Mol Autism 3(1):12. doi:10.1186/2040-2392-3-12
Kostic MS, Rajkovic RJ, Potic Floranovic MS, Dimov ID, Pavlovic D (2013) Multiple sclerosis and oxidative stress—a clinical perspective. Neurochem J 7(1):77–86
Lim CK, Brew BJ, Sundaram G, Guillemin GJ (2010) Understanding the roles of the kynurenine pathway in multiple sclerosis progression. Int J Tryptophan Res 3:157–67
Maes M, Mihaylova I, Ruyter MD, Kubera M, Bosmans E (2007) The immune effects of TRYCATs (tryptophan catabolites along the IDO pathway): relevance for depression—and other conditions characterized by tryptophan depletion induced by inflammation. Neuro Endocrinol Lett 28(6):826–31
Maes M, Berk M, Goehler L, Song C, Anderson G, Gałecki P, Leonard B (2012) Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med 10:66. doi:10.1186/1741-7015-10-66
Maes M, Galecki P, Verkerk R, Rief W (2011) Somatization, but not depression, is characterized by disorders in the tryptophan catabolite (TRYCAT) pathway, indicating increased indoleamine 2,3-dioxygenase and lowered kynurenine aminotransferase activity. Neuro Endocrinol Lett 32(3):264–73
Szalardy L, Klivenyi P, Zadori D, Fulop F, Toldi J, Vecsei L (2012) Mitochondrial disturbances, tryptophan metabolites and neurodegeneration: medicinal chemistry aspects. Curr Med Chem 19(13):1899–920
Kincses ZT, Toldi J, Vécsei L (2010) Kynurenines, neurodegeneration and Alzheimer’s disease. J Cell Mol Med 14(8):2045–54. doi:10.1111/j.1582-4934.2010.01123.x
Brouns R, Verkerk R, Aerts T, De Surgeloose D, Wauters A, Scharpé S, De Deyn PP (2010) The role of tryptophan catabolism along the kynurenine pathway in acute ischemic stroke. Neurochem Res 35(9):1315–22. doi:10.1007/s11064-010-0187-2
Thorne RG, Hanson LR, Ross TM, Tung D, Frey WH 2nd (2008) Delivery of interferon-beta to the monkey nervous system following intranasal administration. Neuroscience 152(3):785–97. doi:10.1016/j.neuroscience.2008.01.013
Malhi GS, Berk M (2007) Does dopamine dysfunction drive depression? Acta PsychiatrScand Suppl 433:116–24
Berk M, Dodd S, Kauer-Sant’anna M, Malhi GS, Bourin M, Kapczinski F, Norman T (2007) Dopamine dysregulation syndrome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr Scand Suppl 434:41–9
Cunnington C, Channon KM (2010) Tetrahydrobiopterin: pleiotropic roles in cardiovascular pathophysiology. Heart 96(23):1872–7. doi:10.1136/hrt.2009.180430
Guillot TS, Miller GW (2009) Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Mol Neurobiol 39(2):149–70. doi:10.1007/s12035-009-8059-y
Riddle EL, Fleckenstein AE, Hanson GR (2006) Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. AAPS J 8(2):E413–8
Kazumori H, Ishihara S, Rumi MA, Ortega-Cava CF, Kadowaki Y, Kinoshita Y (2004) Transforming growth factor-alpha directly augments histidine decarboxylase and vesicular monoamine transporter 2 production in rat enterochromaffin-like cells. Am J Physiol Gastrointest Liver Physiol 286(3):G508–14
Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD (2005) p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem 280(16):15649–58
Zhu CB, Blakely RD, Hewlett WA (2006) The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology 31(10):2121–31
Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA (2010) Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology 35(13):2510–20. doi:10.1038/npp.2010.116
Gautam AH, Zeevalk GD (2011) Characterization of reduced and oxidized dopamine and 3,4-dihydrophenylacetic acid, on brain mitochondrial electron transport chain activities. Biochim Biophys Acta 1807(7):819–28. doi:10.1016/j.bbabio.2011.03.013
Ben-Shachar D (2009) The interplay between mitochondrial complex I, dopamine and Sp1 in schizophrenia. J Neural Transm 116(11):1383–96. doi:10.1007/s00702-009-0319-5
Feier G, Valvassori SS, Lopes-Borges J, Varela RB, Bavaresco DV, Scaini G, Morais MO, Andersen ML, Streck EL, Quevedo J (2012) Behavioral changes and brain energy metabolism dysfunction in rats treated with methamphetamine or dextroamphetamine. Neurosci Lett 530(1):75–9. doi:10.1016/j.neulet.2012.09.039
Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM (2002) Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418(6899):797–801
Angelini C, Bello L, Spinazzi M, Ferrati C (2009) Mitochondrial disorders of the nuclear genome. Acta Myol 28(1):16–23
Jeppesen TD, Schwartz M, Olsen DB, Wibrand F, Krag T, Dunø M, Hauerslev S, Vissing J (2006) Aerobic training is safe and improves exercise capacity in patients with mitochondrial myopathy. Brain 129(Pt 12):3402–12
McFarland R, Turnbull DM (2009) Batteries not included: diagnosis and management of mitochondrial disease. J Intern Med 265(2):210–28. doi:10.1111/j.1365-2796.2008.02066.x
Rose S, Frye RE, Slattery J, Wynne R, Tippett M, Pavliv O, Melnyk S, James SJ (2014) Oxidative stress induces mitochondrial dysfunction in a subset of autism lymphoblastoid cell lines in a well-matched case control cohort. PLoS One 9(1):e85436. doi:10.1371/journal.pone.0085436. eCollection 2014
Sarti P, Forte E, Mastronicola D, Giuffrè A, Arese M (2012) Cytochrome c oxidase and nitric oxide in action: molecular mechanisms and pathophysiological implications. Biochim Biophys Acta 1817(4):610–9. doi:10.1016/j.bbabio.2011.09.002
Leavesley HB, Li L, Prabhakaran K, Borowitz JL, Isom GE (2008) Interaction of cyanide and nitric oxide with cytochrome c oxidase: implications for acute cyanide toxicity. Toxicol Sci 101(1):101–11
Brown GC, Borutaite V (2004) Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim Biophys Acta 1658(1–2):44–9
Maes M (2009) Inflammatory and oxidative and nitrosative stress pathways underpinning chronic fatigue, somatization and psychosomatic symptoms. Curr Opin Psychiatry 22(1):75–83
Vermeulen RC, Eck IW V v (2014) Decreased oxygen extraction during cardiopulmonary exercise test in patients with chronic fatigue syndrome. J Transl Med 12:20. doi:10.1186/1479-5876-12-20
Castro-Marrero J, Cordero MD, Sáez-Francas N, Jimenez-Gutierrez C, Aguilar-Montilla FJ, Aliste L, Alegre-Martin J. Could mitochondrial dysfunction be a differentiating marker between chronic fatigue syndrome and fibromyalgia? Antioxid Redox Signal. 2013 Nov 20;19(15):1855–60. doi: 0.1089/ars.2013.5346. Epub 2013 May 29. PubMed PMID: 23600892.
Alcocer-Gómez E, Sánchez-Alcázar JA, Cordero MD (2014) Coenzyme q10 regulates serotonin levels and depressive symptoms in fibromyalgia patients: results of a small clinical trial. J Clin Psychopharmacol 34(2):277–8. doi:10.1097/JCP.0000000000000097
Cordero, M. D., Cano-García, F. J., Alcocer-Gómez, E., De Miguel, M. and Sánchez-Alcázar, J. A. 2012. Oxidative stress correlates with headache symptoms in fibromyalgia: coenzyme Q10 effect on clinical improvement. PloS one, 7 (4), p. 35677.
Cordero MD, Cotán D, del-Pozo-Martín Y, Carrión AM, de Miguel M, Bullón P, Sánchez-Alcazar JA. Oral coenzyme Q10 supplementation improves clinical symptoms and recovers pathologic alterations in blood mononuclear cells in a fibromyalgia patient. Nutrition. 2012 Nov-Dec;28(11–12):1200–3. doi: 10.1016/j.nut.2012.03.018. Epub 2012 Aug 14. PubMed PMID: 22898267.
Cordero MD, Alcocer-Gómez E, de Miguel M, Culic O, Carrión AM, Alvarez-Suarez JM, Bullón P, Battino M, Fernández-Rodríguez A, Sánchez-Alcazar JA. Can coenzyme q10 improve clinical and molecular parameters in fibromyalgia? Antioxid Redox Signal. 2013 Oct 20;19(12):1356–61. doi: 0.1089/ars.2013.5260. Epub 2013 Apr 6. PubMed PMID: 23458405.
Morris G, Anderson G, Berk M, Maes M (2013) Coenzyme Q10 depletion in medical and neuropsychiatric disorders: potential repercussions and therapeutic implications. Mol Neurobiol 48(3):883–903. doi:10.1007/s12035-013-8477-8
Gökbel H, Gül I, Belviranl M, Okudan N (2010) The effects of coenzyme Q10 supplementation on performance during repeated bouts of supramaximal exercise in sedentary men. J Strength Cond Res 24(1):97–102
Cooke M, Iosia M, Buford T, Shelmadine B, Hudson G, Kerksick C, Rasmussen C, Greenwood M, Leutholtz B, Willoughby D, Kreider R (2008) Effects of acute and 14-day coenzyme Q10 supplementation on exercise performance in both trained and untrained individuals. J Int Soc Sports Nutr 5:8
Alehagen U, Johansson P, Björnstedt M, Rosén A, Dahlström U (2013) Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int J Cardiol 167(5):1860–6. doi:10.1016/j.ijcard.2012.04.156
Finck BN, Kelly DP (2007) Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation 115(19):2540–8
Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, Spiegelman BM (2005) Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab 1(4):259–71
Eschbach J, Schwalenstöcker B, Soyal SM, Bayer H, Wiesner D, Akimoto C, Nilsson AC, Birve A, Meyer T, Dupuis L, Danzer KM, Andersen PM, Witting A, Ludolph AC, Patsch W, Weydt P (2013) PGC-1α is a male-specific disease modifier of human and experimental amyotrophic lateral sclerosis. Hum Mol Genet 22(17):3477–84. doi:10.1093/hmg/ddt202
Tsunemi T, La Spada AR (2012) PGC-1α at the intersection of bioenergetics regulation and neuron function: from Huntington’s disease to Parkinson’s disease and beyond. Prog Neurobiol 97(2):142–51. doi:10.1016/j.pneurobio.2011.10.004
Witte ME, Nijland PG, Drexhage JA, Gerritsen W, Geerts D, van Het Hof B, Reijerkerk A, de Vries HE, van der Valk P, van Horssen J (2013) Reduced expression of PGC-1α partly underlies mitochondrial changes and correlates with neuronal loss in multiple sclerosis cortex. Acta Neuropathol 125(2):231–43. doi:10.1007/s00401-012-1052-y
Summermatter S, Santos G, Pérez-Schindler J, Handschin C (2013) Skeletal muscle PGC-1α controls whole-body lactate homeostasis through estrogen-related receptor α-dependent activation of LDH B and repression of LDH A. Proc Natl Acad Sci U S A 110(21):8738–43. doi:10.1073/pnas.1212976110
Selsby JT, Morine KJ, Pendrak K, Barton ER, Sweeney HL (2012) Rescue of dystrophic skeletal muscle by PGC-1α involves a fast to slow fiber type shift in the mdx mouse. PLoS One 7(1):e30063. doi:10.1371/journal.pone.0030063
Summermatter S, Shui G, Maag D, Santos G, Wenk MR, Handschin C (2013) PGC-1α improves glucose homeostasis in skeletal muscle in an activity-dependent manner. Diabetes 62(1):85–95. doi:10.2337/db12-0291
Bonen A (2009) PGC-1alpha-induced improvements in skeletal muscle metabolism and insulin sensitivity. Appl Physiol Nutr Metab 34(3):307–14. doi:10.1139/H09-008
Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP (2005) PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3(4):e101
Yamada T, Ivarsson N, Hernández A, Fahlström A, Cheng AJ, Zhang SJ, Bruton JD, Ulfhake B, Westerblad H (2012) Impaired mitochondrial respiration and decreased fatigue resistance followed by severe muscle weakness in skeletal muscle of mitochondrial DNA mutator mice. J Physiol 590(Pt 23):6187–97. doi:10.1113/jphysiol.2012.240077
Jung HY, Lee AN, Song TJ, An HS, Kim YH, Kim KD, Kim IB, Kim KS, Han BS, Kim CH, Kim KS, Kim JB (2012) Korean mistletoe (Viscum album coloratum) extract improves endurance capacity in mice by stimulating mitochondrial activity. J Med Food 15(7):621–8. doi:10.1089/jmf.2010.1469
Handschin C, Spiegelman BM (2008) The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 454(7203):463–9. doi:10.1038/nature07206
Tang K, Wagner PD, Breen EC (2010) TNF-alpha-mediated reduction in PGC-1alpha may impair skeletal muscle function after cigarette smoke exposure. J Cell Physiol 222(2):320–7. doi:10.1002/jcp.21955
Palomer X, Alvarez-Guardia D, Rodríguez-Calvo R, Coll T, Laguna JC, Davidson MM, Chan TO, Feldman AM, Vázquez-Carrera M (2009) TNF-alpha reduces PGC-1alpha expression through NF-kappaB and p38 MAPK leading to increased glucose oxidation in a human cardiac cell model. Cardiovasc Res 81(4):703–12. doi:10.1093/cvr/cvn327
Austin S, St-Pierre J (2012) PGC1α and mitochondrial metabolism—emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci 125(Pt 21):4963–71. doi:10.1242/jcs.113662
Tufekci KU, Civi Bayin E, Genc S, Genc K (2011) The Nrf2/ARE pathway: a promising target to counteract mitochondrial dysfunction in Parkinson’s disease. Parkinsons Dis 2011:314082. doi:10.4061/2011/314082
Cook DB, O’Connor PJ, Lange G, Steffener J (2007) Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. Neuroimage 36(1):108–22
Tajima S, Yamamoto S, Tanaka M, Kataoka Y, Iwase M, Yoshikawa E, Okada H, Onoe H, Tsukada H, Kuratsune H, Ouchi Y, Watanabe Y (2010) Medial orbitofrontal cortex is associated with fatigue sensation. Neurol Res Int 2010:671421. doi:10.1155/2010/671421
Chaudhuri A, Behan PO (2004) Fatigue in neurological disorders. Lancet 363(9413):978–88
Bruno RL, Cohen JM, Galski T, Frick NM (1994) The neuroanatomy of post-polio fatigue. Arch Phys Med Rehabil 75(5):498–504
Blomstrand E, Perrett D, Parry-Billings M, Newsholme EA (1989) Effect of sustained exercise on plasma amino acid concentrations and on 5-hydroxytryptamine metabolism in six different brain regions in the rat. Acta Physiol Scand 136(3):473–81
Juengling FD, Ebert D, Gut O, Engelbrecht MA, Rasenack J, Nitzsche EU, Bauer J, Lieb K (2000) Prefrontal cortical hypometabolism during low-dose interferon alpha treatment. Psychopharmaco (Berl) 152(4):383–9
Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, Berns GS, Nemeroff CB, Miller AH (2007) Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology 32(11):2384–92
Eidelberg D, Moeller JR, Dhawan V, Spetsieris P, Takikawa S, Ishikawa T, Chaly T, Robeson W, Margouleff D, Przedborski S et al (1994) The metabolic topography of parkinsonism. J Cereb Blood Flow Metab 14(5):783–801
Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD (2008) Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry 63(11):1022–9. doi:10.1016/j.biopsych.2007.12.007
Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR (2010) Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry 68(8):748–54. doi:10.1016/j.biopsych.2010.06.010
Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL (2005) Neuroanatomical correlates of behavioural disorders in dementia. Brain 128(Pt 11):2612–25
Sepulcre J, Masdeu JC, Goñi J, Arrondo G, Vélez de Mendizábal N, Bejarano B, Villoslada P (2009) Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Mult Scler 15(3):337–44. doi:10.1177/1352458508098373
Okada T, Tanaka M, Kuratsune H, Watanabe Y, Sadato N (2004) Mechanisms underlying fatigue: a voxel-based morphometric study of chronic fatigue syndrome. BMC Neurol 4(1):14
de Lange FP, Koers A, Kalkman JS, Bleijenberg G, Hagoort P, van der Meer JW, Toni I (2008) Increase in prefrontal cortical volume following cognitive behavioural therapy in patients with chronic fatigue syndrome. Brain 131(Pt 8):2172–80. doi:10.1093/brain/awn140
DeLuca J, Genova HM, Hillary FG, Wylie G (2008) Neural correlates of cognitive fatigue in multiple sclerosis using functional MRI. J Neurol Sci 270(1–2):28–39. doi:10.1016/j.jns.2008.01.018
Flachenecker P, Kümpfel T, Kallmann B, Gottschalk M, Grauer O, Rieckmann P, Trenkwalder C, Toyka KV (2002) Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Mult Scler 8(6):523–6
Pardini M, Krueger F, Raymont V, Grafman J (2010) Ventromedial prefrontal cortex modulates fatigue after penetrating traumatic brain injury. Neurology 74(9):749–54. doi:10.1212/WNL.0b013e3181d25b6b
Pardini M, Bonzano L, Roccatagliata L, Mancardi GL, Bove M (2013) The fatigue-motor performance paradox in multiple sclerosis. Sci Rep 3:2001. doi:10.1038/srep02001
Pellicano C, Gallo A, Li X, Ikonomidou VN, Evangelou IE, Ohayon JM, Stern SK, Ehrmantraut M, Cantor F, McFarland HF, Bagnato F (2010) Relationship of cortical atrophy to fatigue in patients with multiple sclerosis. Arch Neurol 67(4):447–53. doi:10.1001/archneurol.2010.48
Cook DB, O’Connor PJ, Lange G, Steffener J (2007) Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. Neuroimage 36(1):108–22
Caseras X, Mataix-Cols D, Rimes KA, Giampietro V, Brammer M, Zelaya F, Chalder T, Godfrey E (2008) The neural correlates of fatigue: an exploratory imaginal fatigue provocation study in chronic fatigue syndrome. Psychol Med 38(7):941–51. doi:10.1017/S0033291708003450
Costa DC, Tannock C, Brostoff J (1995) Brainstem perfusion is impaired in chronic fatigue syndrome. QJM 88:767–773
Ichise M, Salit I, Abbey S, Chung DG, Gray B, Kirsh JC, Freedman M (1992) Assessment of regional cerebral perfusion by Tcm-HMPAO SPECT in chronic fatigue syndrome. Nucl Med Commun 13:767–772
Inglese M, Park SJ, Johnson G, Babb JS, Miles L, Jaggi H, Herbert J, Grossman RI (2007) Deep gray matter perfusion in multiple sclerosis: dynamic susceptibility contrast perfusion magnetic resonance imaging at 3 T. Arch Neurol 64(2):196–202
Niepel G, Tench CR, Morgan PS, Evangelou N, Auer DP, Constantinescu CS (2006) Deep gray matter and fatigue in MS: a T1 relaxation time study. J Neurol 253(7):896–902
Lajoie C, Levasseur MA, Paquet N (2013) Complete normalization of severe brain 18F-FDG hypometabolism following electroconvulsive therapy in a major depressive episode. Clin Nucl Med 38(9):735–6. doi:10.1097/RLU.0b013e31829b9bd9
Thorpe SJ, Rolls ET, Maddison S (1983) The orbitofrontal cortex: neuronal activity in the behaving monkey. Exp Brain Res 49(1):93–115
Walton ME, Bannerman DM, Rushworth MF (2002) The role of rat medial frontal cortex in effort-based decision making. J Neurosci 22(24):10996–1003
Ongür D, An X, Price JL (1998) Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol 401(4):480–505
Carmichael ST, Price JL (1996) Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol 371(2):179–207
Stockton K, Iah K, Paratz DJ, Bennell K (2012) Fatigue, muscle strength and vitamin D status in women with systemic lupus erythematosus compared with healthy controls. Lupus 21(3):271–278
Tench C, Bentley D, Vleck V, Mccurdie I, White P, D’cruz D (2002) Aerobic fitness, fatigue, and physical disability in systemic lupus erythematosus. J rheumatology 29(3):474–481
Kanellopoulos P, Baltoyiannis C, Tzioufas A (2002) Primary Sjögren’s syndrome associated with inclusion body myositis. Rheumatology 41(4):440–444
Vrethem M, Lindvall B, Holmgren H, Henriksson K, Lindström F, Ernerudh J (1990) Neuropathy and myopathy in primary Sjögren’s syndrome: neurophysiological, immunological and muscle biopsy results. Acta Neurol Scand 82(2):126–131
Willer, B., Stucki, G., Hoppeler, H., Brühlmann, P. and Krähenbühl, S. 2000. Effects of creatine supplementation on muscle weakness in patients with rheumatoid arthritis. Rheumatology, 39 (3), pp. 293–298
Yates D (1963) Muscular changes in rheumatoid arthritis. Ann Rheum Dis 22(5):342–347
Friedman JH, Abrantes AM (2012) Self perceived weakness in Parkinson’s disease. Parkinsonism Relat Disord 18(7):887–889
Stevens-Lapsley J, Kluger BM, Schenkman M (2012) Quadriceps muscle weakness, activation deficits, and fatigue with Parkinson disease. Neurorehabil Neural Repair 26(5):533–541
De Haan A, De Ruiter CJ, Van Der Woude LH, Jongen PJ (2000) Contractile properties and fatigue of quadriceps muscles in multiple sclerosis. Muscle Nerve 23(10):1534–1541
Steens A, Heersema D, Maurits NM, Renken R, Zijdewind I (2012) Mechanisms underlying muscle fatigue differ between multiple sclerosis patients and controls: a combined electrophysiological and neuroimaging study. Neuroimage 59(4):3110–3118
Steens A, de Vries A, Hemmen J, Heersema T, Heerings M, Maurits N, Zijdewind I (2012) Fatigue perceived by multiple sclerosis patients is associated with muscle fatigue. Neurorehabil Neural Repair 26(1):48–57. doi:10.1177/1545968311416991
Bandak E, Amris K, Bliddal H, Danneskiold-SamsOe B, Henriksen M (2013) Muscle fatigue in fibromyalgia is in the brain, not in the muscles: a case–control study of perceived versus objective muscle fatigue. Ann Rheum Dis 72(6):963–966
Casale, R., Sarzi-Puttini, P., Atzeni, F., Gazzoni, M., Buskila, D. and Rainoldi, A. 2009. Central motor control failure in fibromyalgia: a surface electromyography study. BMC musculoskeletal disorders, 10 (1), p. 78.
Klaver-Kr’Ol E, Zwarts M, Ten Klooster P, Rasker J (2012) Abnormal muscle membrane function in fibromyalgia patients and its relationship to the number of tender points. Clin Exp Rheumatol 30(6 suppl 74):44–50
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81(4):1725–89
Søgaard K, Gandevia SC, Todd G, Petersen NT, Taylor JL (2006) The effect of sustained low-intensity contractions on supraspinal fatigue in human elbow flexor muscles. J Physiol 573(Pt 2):511–23
Gandevia SC, Allen GM, Butler JE, Taylor JL (1996) Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol 490(Pt 2):529–36
Ranieri F, Di Lazzaro V (2012) The role of motor neuron drive in muscle fatigue. Neuromuscul Disord 22(Suppl 3):S157–61. doi:10.1016/j.nmd.2012.10.006
Taylor JL, Todd G, Gandevia SC (2006) Evidence for a supraspinal contribution to human muscle fatigue. Clin Exp Pharmacol Physiol 33:400–405
Amann M (2012) Significance of group III and IV muscle afferents for the endurance exercising human. Proceedings of the Australian Physiological Society 43:1–7
Mense S, Meyer H (1985) Different types of slowly conducting afferent units in cat skeletal muscle and tendon. J Physiol 363:403–17
Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA (2011) Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol 589(Pt 21):5299–309. doi:10.1113/jphysiol.2011.213769
Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol (1985). 2010 Oct;109(4):966–76. doi: 10.1152/japplphysiol.00462.2010. Epub 2010 Jul 15. PubMed PMID: 20634355; PubMed Central PMCID: PMC2963332.
Tanaka M, Watanabe Y. Supraspinal regulation of physical fatigue. Neurosci Biobehav Rev. 2012 Jan;36(1):727–34. doi: 0.1016/j.neubiorev.2011.10.004. Epub 2011 Oct 25. Review. PubMed PMID: 22040772.
Hilty L, Jäncke L, Luechinger R, Boutellier U, Lutz K (2011) Limitation of physical performance in a muscle fatiguing handgrip exercise is mediated by thalamo-insular activity. Hum Brain Mapp 32(12):2151–60. doi:10.1002/hbm.21177
St Clair Gibson A, Noakes TD. Evidence for complex system integration and dynamic neural regulation of skeletal muscle recruitment during exercise in humans. Br J Sports Med. 2004 Dec;38(6):797–806. Review. PubMed PMID: 15562183; PubMed Central PMCID: PMC1724966.
Noakes TD (2007) The central governor model of exercise regulation applied to the marathon. Sports Med 37(4–5):374–7
Fulle S, Belia S, Vecchiet J, Morabito C, Vecchiet L, Fanò G (2003) Modification of the functional capacity of sarcoplasmic reticulum membranes in patients suffering from chronic fatigue syndrome. Neuromuscul Disord 13(6):479–484
Goldstein D, Bennett B, Friedlander M, Davenport T, Hickie I, Lloyd A (2006) Fatigue states after cancer treatment occur both in association with, and independent of, mood disorder: a longitudinal study. BMC Cancer 6:240
Banerjee M, Siddique S, Dutta A, Mukherjee B, Ranjan Ray M (2012) Cooking with biomass increases the risk of depression in pre-menopausal women in India. Soc Sci Med 75(3):565–72. doi:10.1016/j.socscimed.2012.03.021
Sejersted OM, Sjøgaard G (2000) Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev 80(4):1411–1481
Clausen T (2003) Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev 83(4):1269–1324
Nielsen JJ, Kristensen M, Hellsten Y, Bangsbo J, Juel C (2003) Localization and function of ATP-sensitive potassium channels in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 284(2):R558–R563
Street D, Nielsen JJ, Bangsbo J, Juel C (2005) Metabolic alkalosis reduces exercise-induced acidosis and potassium accumulation in human skeletal muscle interstitium. J Physiol 566(Pt 2):481–489
Bailey DM, Davies B, Young IS, Jackson MJ, Davison GW, Isaacson R, Richardson RS (2003) EPR spectroscopic detection of free radical outflow from an isolated muscle bed in exercising humans. J Appl Physiol 94(5):1714–1718
McKenna MJ, Medved I, Goodman CA, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X (2006) N-acetylcysteine attenuates the decline in muscle Na+, K + −pump activity and delays fatigue during prolonged exercise in humans. J Physiol 576(Pt 1):279–288
Petrushanko I, Bogdanov N, Bulygina E, Grenacher B, Leinsoo T, Boldyrev A, Gassmann M, Bogdanova A (2006) Na-K-ATPase in rat cerebellar granule cells is redox sensitive. Am J Physiol Regul Integr Comp Physiol 290(4):R916–25
Kurella EG, Tyulina OV, Boldyrev AA (1999) Oxidative resistance of Na/K-ATPase. Cell Mol Neurobiol 19(1):133–40
Aughey RJ, Gore CJ, Hahn AG, Garnham AP, Clark SA, Petersen AC, Roberts AD, McKenna MJ. Chronic intermittent hypoxia and incremental cycling exercise independently depress muscle in vitro maximal Na-K+-ATPase activity in well-trained athletes. J Appl Physiol (1985). 2005 Jan;98(1):186–92. Epub 2004 Mar 19. PubMed PMID: 15033968.
Kim MS, Akera T (1987) O2 free radicals: cause of ischemia-reperfusion injury to cardiac Na+-K+-ATPase. Am J Physiol 252(2 Pt 2):H252–H257
Boldyrev AA, Bulygina ER (1997) Na/K-ATPase and oxidative stress. Ann N Y Acad Sci 834:666–668
Vinnikova AK, Kukreja RC, Hess ML (1992) Singlet oxygen-induced inhibition of cardiac sarcolemmal Na+K(+)-ATPase. J Mol Cell Cardiol 24(5):465–470
Cobley JN, McGlory C, Morton JP, Close GL (2011) N-Acetylcysteine’s attenuation of fatigue after repeated bouts of intermittent exercise: practical implications for tournament situations. Int J Sport Nutr Exerc Metab 21(6):451–61
Lai ZW, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, Miklossy G, Jimah J, Doherty E, Tily H, Francis L, Garcia R, Dawood M, Yu J, Ramos I, Coman I, Faraone SV, Phillips PE, Perl A (2012) N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 64(9):2937–46. doi:10.1002/art.34502
Stanislaus R, Gilg AG, Singh AK, Singh I (2005) N-acetyl-l-cysteine ameliorates the inflammatory disease process in experimental autoimmune encephalomyelitis in Lewis rats. J Autoimmune Dis 2(1):4
Jackson WM, Aragon AB, Djouad F, Song Y, Koehler SM, Nesti LJ, Tuan RS (2009) Mesenchymal progenitor cells derived from traumatized human muscle. J Tissue Eng Regen Med 3(2):129–38. doi:10.1002/term.149
Musarò A, Fulle S, Fanò G (2010) Oxidative stress and muscle homeostasis. Curr Opin Clin Nutr Metab Care 13(3):236–42. doi:10.1097/MCO.0b013e3283368188
Naves LA, McCleskey EW (2005) An acid-sensing ion channel that detects ischemic pain. Braz J Med Biol Res 38(11):1561–9
Yagi J, Wenk HN, Naves LA, McCleskey EW (2006) Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res 99(5):501–9
Connor M, Naves LA, McCleskey EW (2005) Contrasting phenotypes of putative proprioceptive and nociceptive trigeminal neurons innervating jaw muscle in rat. Mol Pain 1:31
Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J (2008) Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100:1184–1201
Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A (1994) The chronic fatigue syndrome: a comprehensive approach to its definition and study International Chonic Fatigue Syndrome Study Group. Ann Intern Med 121(12):953–9
Holmes GP, Kaplan JE, Gantz NM, Komaroff AL, Schonberger LB, Straus SE, Jones JF, Dubois RE, Cunningham-Rundles C, Pahwa S et al (1988) Chronic fatigue syndrome: a working case definition. Ann Intern Med 108(3):387–9
Beyer I, Njemini R, Bautmans I, Demanet C, Bergmann P, Mets T (2012) Inflammation-related muscle weakness and fatigue in geriatric patients. Exp Gerontol 47(1):52–9. doi:10.1016/j.exger.2011.10.005
Deshaies RJ, Koch BD, Werner-Washburne M, Craig EA, Schekman R (1988) A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature 332(6167):800–5
Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol (1985). 2002 May;92(5):2177–86. Review. PubMed PMID: 11960972.
Hall TJ (1994) Role of hsp70 in cytokine production. Experientia 50(11–12):1048–53
Moseley P (2000) Stress proteins and the immune response. Immunopharmacology 48(3):299–302
Müller E, Munker R, Issels R, Wilmanns W (1993) Interaction between tumor necrosis factor-alpha and HSP 70 in human leukemia cells. Leuk Res 17(6):523–6
Ensor JE, Wiener SM, McCrea KA, Viscardi RM, Crawford EK, Hasday JD (1994) Differential effects of hyperthermia on macrophage interleukin-6 and tumor necrosis factor-alpha expression. Am J Physiol 266(4 Pt 1):C967–74
Srivastava PK, Udono H, Blachere NE, Li Z (1994) Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics 39(2):93–8
Todryk SM, Melcher AA, Dalgleish AG, Vile RG (2000) Heat shock proteins refine the danger theory. Immunology 99(3):334–7
Locke M (1997) The cellular stress response to exercise: role of stress proteins. Exerc Sport Sci Rev 25:105–36
Thambirajah AA, Sleigh K, Stiver HG, Chow AW (2008) Differential heat shock protein responses to strenuous standardized exercise in chronic fatigue syndrome patients and matched healthy controls. Clin Invest Med 31(6):E319–27
Hooper PL, Hightower LE, Hooper PL (2012) Loss of stress response as a consequence of viral infection: implications for disease and therapy. Cell Stress Chaperones 17(6):647–55. doi:10.1007/s12192-012-0352-4
Liu CC, Lin CH, Lin CY, Lee CC, Lin MT, Wen HC (2013) Transgenic overexpression of heat shock protein 72 in mouse muscle protects against exhaustive exercise-induced skeletal muscle damage. J Formos Med Assoc 112(1):24–30. doi:10.1016/j.jfma.2012.02.007
Kim C, Kim JY, Kim JH (2008) Cytosolic phospholipase A(2), lipoxygenase metabolites, and reactive oxygen species. BMB Rep 41(8):555–9
Vasilaki A, van der Meulen JH, Larkin L, Harrison DC, Pearson T, Van Remmen H, Richardson A, Brooks SV, Jackson MJ, McArdle A (2010) The age-related failure of adaptive responses to contractile activity in skeletal muscle is mimicked in young mice by deletion of Cu, Zn superoxide dismutase. Aging Cell 9(6):979–90. doi:10.1111/j.1474-9726.2010.00635.x
Sen CK, Atalay M, Hänninen O (1994) Exercise-induced oxidative stress: glutathione supplementation and deficiency. J Appl Physiol 77(5):2177–2187
Nakamoto H, Kaneko T, Tahara S, Hayashi E, Naito H, Radak Z, Goto S (2007) Regular exercise reduces 8-oxodG in the nuclear and mitochondrial DNA and modulates the DNA repair activity in the liver of old rats. Exp Gerontol 42(4):287–95
Barreiro E, Gáldiz JB, Mariñán M, Alvarez FJ, Hussain SN, Gea J (2006) Respiratory loading intensity and diaphragm oxidative stress: N-acetyl-cysteine effects. J Appl Physiol 100(2):555–63
Aghdasi B, Zhang JZ, Wu Y, Reid MB, Hamilton SL (1997) Multiple classes of sulfhydryls modulate the skeletal muscle Ca2+ release channel. J Biol Chem 272(6):3739–48
Xu KY, Zweier JL, Becker LC (1997) Hydroxyl radical inhibits sarcoplasmic reticulum Ca(2+)-ATPase function by direct attack on the ATP binding site. Circ Res 80(1):76–81
Putkey JA, Dotson DG, Mouawad P (1993) Formation of inter- and intramolecular disulfide bonds can activate cardiac troponin C. J Biol Chem 268(10):6827–30
Williams DL Jr, Swenson CA (1982) Disulfide bridges in tropomyosin. Effect on ATPase activity of actomyosin Eur J Biochem 127(3):495–9
Ajtai K, Burghardt TP (1989) Fluorescent modification and orientation of myosin sulfhydryl 2 in skeletal muscle fibers. Biochemistry 28(5):2204–10
DalleDonne I, Milzani A, Colombo R (1995) H2O2-treated actin: assembly and polymer interactions with cross-linking proteins. Biophys J 69(6):2710–9
Boldyrev A, Kurella E (1996) Mechanism of oxidative damage of dog kidney Na/K-ATPase. Biochem Biophys Res Commun 222(2):483–7
Funding and competing interests
No specific funding was obtained for this specific review.
GM, MB, PG, and MM declare that they have no competing interests.
Authors’ contributions
GM and MM participated in the design of this review, while PG and MB helped to draft the paper. All authors read and approved the final version.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morris, G., Berk, M., Galecki, P. et al. The Neuro-Immune Pathophysiology of Central and Peripheral Fatigue in Systemic Immune-Inflammatory and Neuro-Immune Diseases. Mol Neurobiol 53, 1195–1219 (2016). https://doi.org/10.1007/s12035-015-9090-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9090-9