Abstract
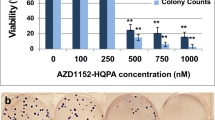
Aurora kinase B (AURKB), a crucial regulator of malignant mitosis, is involved in chromosome segregation and cytokinesis. AZD1152-HQPA is a selective inhibitor for AURKB activity and currently bears clinical assessment for several malignancies. However, the effect of this drug still needs to be elucidated in neurological malignancies. In this study, we investigated the restrictive potentials of AZD1152-HQPA in U87MG and SK-N-MC. AZD1152-HQPA treatment resulted in growth arrest, modification of cell cycle, and inhibition of colony formation in both cell lines. Furthermore, lower concentrations of AZD1152-HQPA profoundly induced apoptosis in U87GM (p53/p73 wild type) cells in parallel with an upregulation of p53 and its target genes BAX, BAD, APAF1, and PUMA. But remarkably, SK-N-MC (p53/p73 double null) responded to AZD1152-HQPA at much higher concentrations with an upregulation of genes involved in cell cycle progression, induction of excessive endoreduplication, and polyploidy rather than apoptosis. Although SK-N-MC was resistant to AZD1152-HQPA, we did not find a mutation in the coding sequence of Aurora B gene or overexpressions of ABCG2 and ABCB1 as reported previously to be resistance mechanisms. However, our results suggest that p53/p73 status could be an important mechanism for the type of response and resistance of the tumor cells to AZD1152-HQPA. Collectively, inhibition of Aurora kinase B differentially induced cell death and polyploidy via DNA damage response pathways, depending on the status of p53/p73. We suggest p53/p73 could be a key regulator of sensitivity to AZD1152-HQPA and their status should be explored in clinical response to this ongoing drug in clinical trials.










Similar content being viewed by others
References
Khasraw M, Lassman AB (2010) Advances in the treatment of malignant gliomas. Curr Oncol Rep 12(1):26–33. doi:10.1007/s11912-009-0077-4
Brodeur GM (2003) Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer 3(3):203–216. doi:10.1038/nrc1014
Hontz AE, Li SA, Lingle WL, Negron V, Bruzek A, Salisbury JL, Li JJ (2007) Aurora A and B overexpression and centrosome amplification in early estrogen-induced tumor foci in the Syrian hamster kidney: implications for chromosomal instability, aneuploidy, and neoplasia. Cancer Res 67(7):2957–2963
Carmena M, Earnshaw WC (2003) The cellular geography of aurora kinases. Nat Rev 4(11):842–854
Zeng WF, Navaratne K, Prayson RA, Weil RJ (2007) Aurora B expression correlates with aggressive behaviour in glioblastoma multiforme. J Clin Pathol 60(2):218–221. doi:10.1136/jcp.2006.036806
Chieffi P, Cozzolino L, Kisslinger A, Libertini S, Staibano S, Mansueto G, De Rosa G, Villacci A et al (2006) Aurora B expression directly correlates with prostate cancer malignancy and influence prostate cell proliferation. Prostate 66(3):326–333. doi:10.1002/pros.20345
Li D, Zhu J, Firozi PF, Abbruzzese JL, Evans DB, Cleary K, Friess H, Sen S (2003) Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin Cancer Res 9(3):991–997
Ulisse S, Delcros JG, Baldini E, Toller M, Curcio F, Giacomelli L, Prigent C, Ambesi-Impiombato FS et al (2006) Expression of Aurora kinases in human thyroid carcinoma cell lines and tissues. Int J Cancer 119(2):275–282. doi:10.1002/ijc.21842
Ikezoe T, Yang J, Nishioka C, Tasaka T, Taniguchi A, Kuwayama Y, Komatsu N, Bandobashi K et al (2007) A novel treatment strategy targeting Aurora kinases in acute myelogenous leukemia. Mol Cancer Ther 6(6):1851–1857. doi:10.1158/1535-7163.MCT-07-0067
Michaelis M, Selt F, Rothweiler F, Loschmann N, Nusse B, Dirks WG, Zehner R, Cinatl J Jr (2014) Aurora kinases as targets in drug-resistant neuroblastoma cells. PLoS One 9(9):e108758. doi:10.1371/journal.pone.0108758
Borges KS, Castro-Gamero AM, Moreno DA, da Silva SV, Brassesco MS, de Paula Queiroz RG, de Oliveira HF, Carlotti CG Jr et al (2012) Inhibition of Aurora kinases enhances chemosensitivity to temozolomide and causes radiosensitization in glioblastoma cells. J Cancer Res Clin Oncol 138(3):405–414. doi:10.1007/s00432-011-1111-0
Morozova O, Vojvodic M, Grinshtein N, Hansford LM, Blakely KM, Maslova A, Hirst M, Cezard T et al (2010) System-level analysis of neuroblastoma tumor-initiating cells implicates AURKB as a novel drug target for neuroblastoma. Clin Cancer Res 16(18):4572–4582. doi:10.1158/1078-0432.CCR-10-0627
Van Brocklyn JR, Wojton J, Meisen WH, Kellough DA, Ecsedy JA, Kaur B, Lehman NL (2014) Aurora-A inhibition offers a novel therapy effective against intracranial glioblastoma. Cancer Res 74(19):5364–5370. doi:10.1158/0008-5472.CAN-14-0386
Hong X, O’Donnell JP, Salazar CR, Van Brocklyn JR, Barnett KD, Pearl DK, deCarvalho AC, Ecsedy JA et al (2014) The selective Aurora-A kinase inhibitor MLN8237 (alisertib) potently inhibits proliferation of glioblastoma neurosphere tumor stem-like cells and potentiates the effects of temozolomide and ionizing radiation. Cancer Chemother Pharmacol 73(5):983–990. doi:10.1007/s00280-014-2430-z
Dennis M, Davies M, Oliver S, D’Souza R, Pike L, Stockman P (2012) Phase I study of the Aurora B kinase inhibitor barasertib (AZD1152) to assess the pharmacokinetics, metabolism and excretion in patients with acute myeloid leukemia. Cancer Chemother Pharmacol 70(3):461–469. doi:10.1007/s00280-012-1939-2
Schwartz GK, Carvajal RD, Midgley R, Rodig SJ, Stockman PK, Ataman O, Wilson D, Das S et al (2012) Phase I study of barasertib (AZD1152), a selective inhibitor of Aurora B kinase, in patients with advanced solid tumors. Invest New Drugs. doi:10.1007/s10637-012-9825-7
Zekri A, Ghaffari SH, Ghanizadeh-Vesali S, Yaghmaie M, Salmaninejad A, Alimoghaddam K, Modarressi MH, Ghavamzadeh A (2014) AZD1152-HQPA induces growth arrest and apoptosis in androgen-dependent prostate cancer cell line (LNCaP) via producing aneugenic micronuclei and polyploidy. Tumour Biol. doi:10.1007/s13277-014-2664-8
Pluim D, Beijnen JH, Schellens JH, van Tellingen O (2009) Simultaneous determination of AZD1152 (prodrug) and AZD1152-hydroxyquinazoline pyrazol anilide by reversed phase liquid chromatography. J Chromatogr B Anal Technol Biomed Life Sci 877(29):3549–3555. doi:10.1016/j.jchromb.2009.08.035
Talos F, Nemajerova A, Flores ER, Petrenko O, Moll UM (2007) p73 suppresses polyploidy and aneuploidy in the absence of functional p53. Mol Cell 27(4):647–659. doi:10.1016/j.molcel.2007.06.036
Aihara A, Tanaka S, Yasen M, Matsumura S, Mitsunori Y, Murakata A, Noguchi N, Kudo A et al (2010) The selective Aurora B kinase inhibitor AZD1152 as a novel treatment for hepatocellular carcinoma. J Hepatol 52(1):63–71. doi:10.1016/j.jhep.2009.10.013
Tao Y, Zhang P, Girdler F, Frascogna V, Castedo M, Bourhis J, Kroemer G, Deutsch E (2008) Enhancement of radiation response in p53-deficient cancer cells by the Aurora-B kinase inhibitor AZD1152. Oncogene 27(23):3244–3255. doi:10.1038/sj.onc.1210990
Freed-Pastor WA, Prives C (2012) Mutant p53: one name, many proteins. Genes Dev 26(12):1268–1286. doi:10.1101/gad.190678.112
Fenech M (2000) The in vitro micronucleus technique. Mutat Res 455(1–2):81–95
Davidoff AM, Pence JC, Shorter NA, Iglehart JD, Marks JR (1992) Expression of p53 in human neuroblastoma- and neuroepithelioma-derived cell lines. Oncogene 7(1):127–133
Gartel AL, Tyner AL (2002) The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther 1(8):639–649
Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A, Minty A, Chalon P et al (1997) Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell 90(4):809–819
Girdler F, Sessa F, Patercoli S, Villa F, Musacchio A, Taylor S (2008) Molecular basis of drug resistance in aurora kinases. Chem Biol 15(6):552–562. doi:10.1016/j.chembiol.2008.04.013
Walsby E, Walsh V, Pepper C, Burnett A, Mills K (2008) Effects of the aurora kinase inhibitors AZD1152-HQPA and ZM447439 on growth arrest and polyploidy in acute myeloid leukemia cell lines and primary blasts. Haematologica 93(5):662–669. doi:10.3324/haematol.12148
Sasai K, Katayama H, Stenoien DL, Fujii S, Honda R, Kimura M, Okano Y, Tatsuka M et al (2004) Aurora-C kinase is a novel chromosomal passenger protein that can complement Aurora-B kinase function in mitotic cells. Cell Motil Cytoskeleton 59(4):249–263. doi:10.1002/cm.20039
Guo J, Anderson MG, Tapang P, Palma JP, Rodriguez LE, Niquette A, Li J, Bouska JJ et al (2009) Identification of genes that confer tumor cell resistance to the aurora B kinase inhibitor, AZD1152. Pharmacogenomics J 9(2):90–102. doi:10.1038/tpj.2008.20
Talos F, Moll UM (2010) Role of the p53 family in stabilizing the genome and preventing polyploidization. Adv Exp Med Biol 676:73–91
Tsuiki H, Nitta M, Tada M, Inagaki M, Ushio Y, Saya H (2001) Mechanism of hyperploid cell formation induced by microtubule inhibiting drug in glioma cell lines. Oncogene 20(4):420–429
Tomasini R, Mak TW, Melino G (2008) The impact of p53 and p73 on aneuploidy and cancer. Trends Cell Biol 18(5):244–252. doi:10.1016/j.tcb.2008.03.003
Stukenberg PT (2004) Triggering p53 after cytokinesis failure. J Cell Biol 165(5):607–608. doi:10.1083/jcb.200405089
Sak A, Stuschke M, Groneberg M, Kubler D, Pottgen C, Eberhardt WE (2012) Inhibiting the aurora B kinase potently suppresses repopulation during fractionated irradiation of human lung cancer cell lines. Int J Radiat Oncol Biol Phys 84(2):492–499. doi:10.1016/j.ijrobp.2011.12.021
Diaz RJ, Golbourn B, Shekarforoush M, Smith CA, Rutka JT (2012) Aurora kinase B/C inhibition impairs malignant glioma growth in vivo. J Neurooncol 108(3):349–360. doi:10.1007/s11060-012-0835-2
Yu J, Zhang H, Gu J, Lin S, Li J, Lu W, Wang Y, Zhu J (2004) Methylation profiles of thirty four promoter-CpG islands and concordant methylation behaviours of sixteen genes that may contribute to carcinogenesis of astrocytoma. BMC Cancer 4:65. doi:10.1186/1471-2407-4-65
Stokes MP, Rush J, Macneill J, Ren JM, Sprott K, Nardone J, Yang V, Beausoleil SA et al (2007) Profiling of UV-induced ATM/ATR signaling pathways. Proc Natl Acad Sci U S A 104(50):19855–19860. doi:10.1073/pnas.0707579104
Vitale I, Galluzzi L, Castedo M, Kroemer G (2011) Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol 12(6):385–392. doi:10.1038/nrm3115
Bassing CH, Alt FW (2004) The cellular response to general and programmed DNA double strand breaks. DNA Repair 3(8–9):781–796
Featherstone C, Jackson SP (1999) DNA double-strand break repair. Curr Biol 9(20):R759–R761
Acknowledgments
We thank AstraZeneca pharmaceutical company for supplying the Aurora B inhibitor AZD1152-HQPA. We thank Azam Zaghal for of flow cytometry analyses and Elham Hossaini and Masomeh Rostami for their help in this study.
Compliance with Ethical Standards
ᅟ
Funding
This study was funded by Hematology, Oncology and stem cell therapy, Tehran University of Medical Sciences, Tehran, Iran (grant number 1619).
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants and/or Animals
This research did not involve any human participants, and no animals were involved in this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zekri, A., Ghaffari, S.H., Yaghmaie, M. et al. Inhibitor of Aurora Kinase B Induces Differentially Cell Death and Polyploidy via DNA Damage Response Pathways in Neurological Malignancy: Shedding New Light on the Challenge of Resistance to AZD1152-HQPA. Mol Neurobiol 53, 1808–1823 (2016). https://doi.org/10.1007/s12035-015-9139-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9139-9