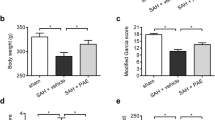
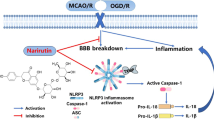
Abstract
Pterostilbene (PTE), one of the polyphenols present in plants such as blueberries and grapes, has been suggested to have various effects, such as anti-oxidation, anti-apoptosis, and anti-cancer effects. Subarachnoid hemorrhage (SAH) is a severe neurological event known for its high morbidity and mortality. Recently, early brain injury (EBI) has been reported to play a significant role in the prognosis of patients with SAH. The present study aimed to investigate whether PTE could attenuate EBI after SAH was induced in C57BL/6 J mice. We also studied possible underlying mechanisms. After PTE treatment, the neurological score and brain water content of the mice were assessed. Oxidative stress and neuronal injury were also evaluated. Nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome activity was assessed using western blot analysis. Our results indicated that PTE treatment reduces the SAH grade, neurological score, and brain water content following SAH. PTE treatment also reduced NLRP3 inflammasome activation. PTE alleviated the oxidative stress following SAH as evidenced by the dihydroethidium staining, superoxide dismutase activity, malondialdehyde content, 3-nitrotyrosie and 8-hydroxy-2-deoxyguanosine levels, and gp91phox and 4-hydroxynonenal expression levels. Additionally, PTE treatment reduced neuronal apoptosis. In conclusion, our study suggests that PTE attenuates EBI following SAH possibly via the inhibition of NLRP3 inflammasome and Nox2-related oxidative stress.






Similar content being viewed by others
References
Sehba FA, Hou J, Pluta RM, Zhang JH (2012) The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol 97(1):14–37. doi:10.1016/j.pneurobio.2012.02.003
Bing Z, Rabinstein AA, Murad MH, Lanzino G, Panni P, Brinjikji W (2015) Surgical and endovascular treatment of poor-grade aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Neurosurg Sci
Nogueira AB, Nogueira AB, Esteves Veiga JC, Teixeira MJ (2014) Multimodality monitoring, inflammation, and neuroregeneration in subarachnoid hemorrhage. Neurosurgery 75(6):678–689. doi:10.1227/NEU.0000000000000512
Chen S, Feng H, Sherchan P, Klebe D, Zhao G, Sun X, Zhang J, Tang J, Zhang JH (2014) Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurobiol 115:64–91. doi:10.1016/j.pneurobio.2013.09.002
Dreier JP, Drenckhahn C, Woitzik J, Major S, Offenhauser N, Weber-Carstens S, Wolf S, Strong AJ, Vajkoczy P, Hartings JA, Group CS (2013) Spreading ischemia after aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl 115:125–129. doi:10.1007/978-3-7091-1192-5_26
Helbok R, Schiefecker AJ, Beer R, Dietmann A, Antunes AP, Sohm F, Fischer M, Hackl WO, Rhomberg P, Lackner P, Pfausler B, Thome C, Humpel C, Schmutzhard E (2015) Early brain injury after aneurysmal subarachnoid hemorrhage: a multimodal neuromonitoring study. Crit Care 19:75. doi:10.1186/s13054-015-0809-9
Echigo R, Shimohata N, Karatsu K, Yano F, Kayasuga-Kariya Y, Fujisawa A, Ohto T, Kita Y, Nakamura M, Suzuki S, Mochizuki M, Shimizu T, Chung UI, Sasaki N (2012) Trehalose treatment suppresses inflammation, oxidative stress, and vasospasm induced by experimental subarachnoid hemorrhage. J Transl Med 10:80. doi:10.1186/1479-5876-10-80
Hernandes MS, D’Avila JC, Trevelin SC, Reis PA, Kinjo ER, Lopes LR, Castro-Faria-Neto HC, Cunha FQ, Britto LR, Bozza FA (2014) The role of Nox2-derived ROS in the development of cognitive impairment after sepsis. J Neuroinflammation 11:36. doi:10.1186/1742-2094-11-36
Lucke-Wold BP, Logsdon AF, Manoranjan B, Turner RC, McConnell E, Vates GE, Huber JD, Rosen CL, Simard JM (2016) Aneurysmal subarachnoid hemorrhage and neuroinflammation: a comprehensive review. Int J Mol Sci 17(4). doi:10.3390/ijms17040497
Zhao L, Liu H, Yue L, Zhang J, Li X, Wang B, Lin Y, Qu Y (2016) Melatonin attenuates early brain injury via the melatonin receptor/Sirt1/NF-kappaB signaling pathway following subarachnoid hemorrhage in mice. Mol Neurobiol. doi:10.1007/s12035-016-9776-7
Shao A, Wu H, Hong Y, Tu S, Sun X, Wu Q, Zhao Q, Zhang J, Sheng J (2015) Hydrogen-rich saline attenuated subarachnoid hemorrhage-induced early brain injury in rats by suppressing inflammatory response: possible involvement of NF-kappaB pathway and NLRP3 inflammasome. Mol Neurobiol. doi:10.1007/s12035-015-9242-y
Lamkanfi M, Dixit VM (2011) Modulation of inflammasome pathways by bacterial and viral pathogens. J Immunol 187(2):597–602. doi:10.4049/jimmunol.1100229
Felderhoff-Mueser U, Schmidt OI, Oberholzer A, Buhrer C, Stahel PF (2005) IL-18: a key player in neuroinflammation and neurodegeneration? Trends Neurosci 28(9):487–493. doi:10.1016/j.tins.2005.06.008
Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL (2011) Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol 68(3):593–601. doi:10.1007/s00280-010-1525-4
Estrela JM, Ortega A, Mena S, Rodriguez ML, Asensi M (2013) Pterostilbene: Biomedical applications. Crit Rev Clin Lab Sci 50(3):65–78. doi:10.3109/10408363.2013.805182
Nikhil K, Sharan S, Palla SR, Sondhi SM, Peddinti RK, Roy P (2015) Understanding the mode of action of a pterostilbene derivative as anti-inflammatory agent. Int Immunopharmacol 28(1):10–21. doi:10.1016/j.intimp.2015.05.003
Huynh TT, Lin CM, Lee WH, Wu AT, Lin YK, Lin YF, Yeh CT, Wang LS (2015) Pterostilbene suppressed irradiation-resistant glioma stem cells by modulating GRP78/miR-205 axis. J Nutr Biochem 26(5):466–475. doi:10.1016/j.jnutbio.2014.11.015
Yang Y, Wang J, Li Y, Fan C, Jiang S, Zhao L, Di S, Xin Z, Wang B, Wu G, Li X, Li Z, Gao X, Dong Y, Qu Y (2015) HO-1 signaling activation by pterostilbene treatment attenuates mitochondrial oxidative damage induced by cerebral ischemia reperfusion injury. Mol Neurobiol. doi:10.1007/s12035-015-9194-2
Sugawara T, Ayer R, Jadhav V, Zhang JH (2008) A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods 167(2):327–334. doi:10.1016/j.jneumeth.2007.08.004
Yan H, Zhang D, Hao S, Li K, Hang CH (2015) Role of mitochondrial calcium uniporter in early brain injury after experimental subarachnoid hemorrhage. Mol Neurobiol 52(3):1637–1647. doi:10.1007/s12035-014-8942-z
Zhang XS, Zhang X, Zhou ML, Zhou XM, Li N, Li W, Cong ZX, Sun Q, Zhuang Z, Wang CX, Shi JX (2014) Amelioration of oxidative stress and protection against early brain injury by astaxanthin after experimental subarachnoid hemorrhage. J Neurosurg 121(1):42–54. doi:10.3171/2014.2.JNS13730
Cai J, Cao S, Chen J, Yan F, Chen G, Dai Y (2015) Progesterone alleviates acute brain injury via reducing apoptosis and oxidative stress in a rat experimental subarachnoid hemorrhage model. Neurosci Lett 600:238–243. doi:10.1016/j.neulet.2015.06.023
Pylvas M, Puistola U, Laatio L, Kauppila S, Karihtala P (2011) Elevated serum 8-OHdG is associated with poor prognosis in epithelial ovarian cancer. Anticancer Res 31(4):1411–1415
Bederson JB, Connolly ES Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, Duldner JE Jr, Harbaugh RE, Patel AB, Rosenwasser RH, American Heart A (2009) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the stroke council, American Heart Association. Stroke; a journal of cerebral circulation 40(3):994–1025. doi:10.1161/STROKEAHA.108.191395
Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A (1994) Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke; J Cereb Circ 25(7):1342–1347
Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH (2013) Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res 4(4):432–446. doi:10.1007/s12975-013-0257-2
Cahill J, Calvert JW, Zhang JH (2006) Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab 26(11):1341–1353. doi:10.1038/sj.jcbfm.9600283
Yuksel S, Tosun YB, Cahill J, Solaroglu I (2012) Early brain injury following aneurysmal subarachnoid hemorrhage: emphasis on cellular apoptosis. Turk Neurosurg 22(5):529–533. doi:10.5137/1019-5149.JTN.5731-12.1
McCormack D, McFadden D (2013) A review of pterostilbene antioxidant activity and disease modification. Oxidative Med Cell Longev 2013:575482. doi:10.1155/2013/575482
Kasiotis KM, Pratsinis H, Kletsas D, Haroutounian SA (2013) Resveratrol and related stilbenes: their anti-aging and anti-angiogenic properties. Food Chem Toxicol: Int J Publ Br Ind Biol Res Assoc 61:112–120. doi:10.1016/j.fct.2013.03.038
Poulose SM, Thangthaeng N, Miller MG, Shukitt-Hale B (2015) Effects of pterostilbene and resveratrol on brain and behavior. Neurochem Int 89:227–233. doi:10.1016/j.neuint.2015.07.017
Rimando AM, Cuendet M, Desmarchelier C, Mehta RG, Pezzuto JM, Duke SO (2002) Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J Agric Food Chem 50(12):3453–3457
Sies H (2010) Polyphenols and health: update and perspectives. Arch Biochem Biophys 501(1):2–5. doi:10.1016/j.abb.2010.04.006
Stevenson DE, Hurst RD (2007) Polyphenolic phytochemicals—just antioxidants or much more? Cell Mol Life Sci: CMLS 64(22):2900–2916. doi:10.1007/s00018-007-7237-1
Chiou YS, Tsai ML, Wang YJ, Cheng AC, Lai WM, Badmaev V, Ho CT, Pan MH (2010) Pterostilbene inhibits colorectal aberrant crypt foci (ACF) and colon carcinogenesis via suppression of multiple signal transduction pathways in azoxymethane-treated mice. J Agric Food Chem 58(15):8833–8841. doi:10.1021/jf101571z
Wang W, Ding XQ, Gu TT, Song L, Li JM, Xue QC, Kong LD (2015) Pterostilbene and allopurinol reduce fructose-induced podocyte oxidative stress and inflammation via microRNA-377. Free Radic Biol Med 83:214–226. doi:10.1016/j.freeradbiomed.2015.02.029
Cairns B, Kim JY, Tang XN, Yenari MA (2012) NOX inhibitors as a therapeutic strategy for stroke and neurodegenerative disease. Curr Drug Targets 13(2):199–206
Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ (2014) NADPH oxidases in vascular pathology. Antioxid Redox Signal 20(17):2794–2814. doi:10.1089/ars.2013.5607
Leto TL, Morand S, Hurt D, Ueyama T (2009) Targeting and regulation of reactive oxygen species generation by Nox Family NADPH oxidases. Antioxid Redox Signal 11(10):2607–2619. doi:10.1089/ARS.2009.2637
Zhang ZY, Sun BL, Yang MF, Li DW, Fang J, Zhang S (2015) Carnosine attenuates early brain injury through its antioxidative and anti-apoptotic effects in a rat experimental subarachnoid hemorrhage model. Cell Mol Neurobiol 35(2):147–157. doi:10.1007/s10571-014-0106-1
Witherell HL, Hiatt RA, Replogle M, Parsonnet J (1998) Helicobacter pylori infection and urinary excretion of 8-hydroxy-2-deoxyguanosine, an oxidative DNA adduct. Cancer Epidemiol Biomark Prev: Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 7(2):91–96
Ayer R, Chen W, Sugawara T, Suzuki H, Zhang JH (2010) Role of gap junctions in early brain injury following subarachnoid hemorrhage. Brain Res 1315:150–158. doi:10.1016/j.brainres.2009.12.016
Ayer R, Jadhav V, Sugawara T, Zhang JH (2011) The neuroprotective effects of cyclooxygenase-2 inhibition in a mouse model of aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl 111:145–149. doi:10.1007/978-3-7091-0693-8_24
Moraes L, Grille S, Morelli P, Mila R, Trias N, Brugnini A, N LL, Biestro A, Lens D (2015) Immune cells subpopulations in cerebrospinal fluid and peripheral blood of patients with aneurysmal subarachnoid hemorrhage. SpringerPlus 4:195. doi:10.1186/s40064-015-0970-2
Yang F, Wang Z, Wei X, Han H, Meng X, Zhang Y, Shi W, Li F, Xin T, Pang Q, Yi F (2014) NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab 34(4):660–667. doi:10.1038/jcbfm.2013.242
Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J (2014) NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol 75(2):209–219. doi:10.1002/ana.24070
Liu HD, Li W, Chen ZR, Hu YC, Zhang DD, Shen W, Zhou ML, Zhu L, Hang CH (2013) Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochem Res 38(10):2072–2083. doi:10.1007/s11064-013-1115-z
Hoegen T, Tremel N, Klein M, Angele B, Wagner H, Kirschning C, Pfister HW, Fontana A, Hammerschmidt S, Koedel U (2011) The NLRP3 inflammasome contributes to brain injury in pneumococcal meningitis and is activated through ATP-dependent lysosomal cathepsin B release. J Immunol 187(10):5440–5451. doi:10.4049/jimmunol.1100790
Jo EK, Kim JK, Shin DM, Sasakawa C (2016) Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 13(2):148–159. doi:10.1038/cmi.2015.95
Denes A, Coutts G, Lenart N, Cruickshank SM, Pelegrin P, Skinner J, Rothwell N, Allan SM, Brough D (2015) AIM2 and NLRC4 inflammasomes contribute with ASC to acute brain injury independently of NLRP3. Proc Natl Acad Sci U S A 112(13):4050–4055. doi:10.1073/pnas.1419090112
Sutterwala FS, Haasken S, Cassel SL (2014) Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci 1319:82–95. doi:10.1111/nyas.12458
Fann DY, Lee SY, Manzanero S, Tang SC, Gelderblom M, Chunduri P, Bernreuther C, Glatzel M, Cheng YL, Thundyil J, Widiapradja A, Lok KZ, Foo SL, Wang YC, Li YI, Drummond GR, Basta M, Magnus T, Jo DG, Mattson MP, Sobey CG, Arumugam TV (2013) Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis 4:e790. doi:10.1038/cddis.2013.326
Abais JM, Xia M, Zhang Y, Boini KM, Li PL (2015) Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal 22(13):1111–1129. doi:10.1089/ars.2014.5994
Zhang X, Zhang JH, Chen XY, Hu QH, Wang MX, Jin R, Zhang QY, Wang W, Wang R, Kang LL, Li JS, Li M, Pan Y, Huang JJ, Kong LD (2015) Reactive oxygen species-induced TXNIP drives fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxid Redox Signal 22(10):848–870. doi:10.1089/ars.2014.5868
Elliott EI, Sutterwala FS (2015) Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev 265(1):35–52. doi:10.1111/imr.12286
Li J, Chen J, Mo H, Chen J, Qian C, Yan F, Gu C, Hu Q, Wang L, Chen G (2016) Minocycline protects against NLRP3 inflammasome-induced inflammation and P53-associated apoptosis in early brain injury after subarachnoid hemorrhage. Mol Neurobiol 53(4):2668–2678. doi:10.1007/s12035-015-9318-8
Katsnelson MA, Lozada-Soto KM, Russo HM, Miller BA, Dubyak GR (2016) NLRP3 inflammasome signaling is activated by low-level lysosome disruption but inhibited by extensive lysosome disruption: roles for K+ efflux and Ca2+ influx. Am J Physiol Cell Physiol 311(1):C83–C100. doi:10.1152/ajpcell.00298.2015
Watters O, O Connor JJ (2011) A role for tumor necrosis factor-alpha in ischemia and ischemic preconditioning. J Neuroinflammation 8:87. doi:10.1186/1742-2094-8-87
Verma G, Bhatia H, Datta M (2013) JNK1/2 regulates ER-mitochondrial Ca2+ cross-talk during IL-1beta-mediated cell death in RINm5F and human primary beta-cells. Mol Biol Cell 24(12):2058–2071. doi:10.1091/mbc.E12-12-0885
Nakka VP, Gusain A, Mehta SL, Raghubir R (2008) Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Mol Neurobiol 37(1):7–38. doi:10.1007/s12035-007-8013-9
Li T, Liu H, Xue H, Zhang J, Han X, Yan S, Bo S, Liu S, Yuan L, Deng L, Li G, Wang Z (2016) Neuroprotective effects of hydrogen sulfide against early brain injury and secondary cognitive deficits following subarachnoid hemorrhage. Brain Pathol. doi:10.1111/bpa.12361
Luna-Vargas MP, Chipuk JE (2016) Physiological and pharmacological control of BAK, BAX, and beyond. Trends Cell Biol. doi:10.1016/j.tcb.2016.07.002
Suzuki M, Youle RJ, Tjandra N (2000) Structure of Bax: coregulation of dimer formation and intracellular localization. Cell 103(4):645–654
Zhang Z, Lapolla SM, Annis MG, Truscott M, Roberts GJ, Miao Y, Shao Y, Tan C, Peng J, Johnson AE, Zhang XC, Andrews DW, Lin J (2004) Bcl-2 homodimerization involves two distinct binding surfaces, a topographic arrangement that provides an effective mechanism for Bcl-2 to capture activated Bax. J Biol Chem 279(42):43920–43928. doi:10.1074/jbc.M406412200
Engel T, Plesnila N, Prehn JH, Henshall DC (2011) In vivo contributions of BH3-only proteins to neuronal death following seizures, ischemia, and traumatic brain injury. J Cereb Blood Flow Metab: Official J Int Soc Cereb Blood Flow Metab 31(5):1196–1210. doi:10.1038/jcbfm.2011.26
Chong SJ, Low IC, Pervaiz S (2014) Mitochondrial ROS and involvement of Bcl-2 as a mitochondrial ROS regulator. Mitochondrion 19:39–48. doi:10.1016/j.mito.2014.06.002
Jemmerson R, Dubinsky JM, Brustovetsky N (2005) Cytochrome C release from CNS mitochondria and potential for clinical intervention in apoptosis-mediated CNS diseases. Antioxid Redox Signal 7(9–10):1158–1172. doi:10.1089/ars.2005.7.1158
Jang JH, Surh YJ (2003) Potentiation of cellular antioxidant capacity by Bcl-2: implications for its antiapoptotic function. Biochem Pharmacol 66(8):1371–1379
Zhou XM, Zhou ML, Zhang XS, Zhuang Z, Li T, Shi JX, Zhang X (2014) Resveratrol prevents neuronal apoptosis in an early brain injury model. J Surg Res 189(1):159–165. doi:10.1016/j.jss.2014.01.062
Hao XK, Wu W, Wang CX, Xie GB, Li T, Wu HM, Huang LT, Zhou ML, Hang CH, Shi JX (2014) Ghrelin alleviates early brain injury after subarachnoid hemorrhage via the PI3K/Akt signaling pathway. Brain Res 1587:15–22. doi:10.1016/j.brainres.2014.08.069
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, Thompson BG, Vespa P, American Heart Association Stroke Council, Council on Cardiovascular Radiology and Intervention, Council on Cardiovascular Nursing, Council on Cardiovascular Surgery and Anesthesia, Council on Clinical Cardiology (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke: J Cereb Circ 43(6):1711–1737. doi:10.1161/STR.0b013e3182587839
Behrouz R, Sadat-Hosseiny Z (2015) Pharmacological agents in aneurysmal subarachnoid hemorrhage: successes and failures. Clin Neuropharmacol 38(3):104–108. doi:10.1097/WNF.0000000000000085
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81571215, 81630027, 81401020) and Leading Talents of Middle Age and Young in S & T Innovation supported by the Chinese Science and Technology Ministry (2013RA2181).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have declared that no competing interests exist.
Additional information
Haixiao Liu, Lei Zhao, and Liang Yue contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, H., Zhao, L., Yue, L. et al. Pterostilbene Attenuates Early Brain Injury Following Subarachnoid Hemorrhage via Inhibition of the NLRP3 Inflammasome and Nox2-Related Oxidative Stress. Mol Neurobiol 54, 5928–5940 (2017). https://doi.org/10.1007/s12035-016-0108-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0108-8