Abstract
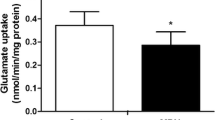
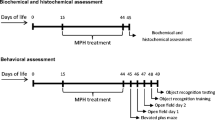
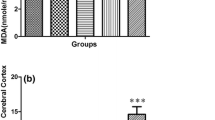
The study of the long-term neurological consequences of early exposure with methylphenidate (MPH) is very important since this psychostimulant has been widely misused by children and adolescents who do not meet full diagnostic criteria for ADHD. The aim of this study was to examine the effect of early chronic exposure with MPH on amino acids profile, glutamatergic and Na+,K+-ATPase homeostasis, as well as redox and energy status in the hippocampus of juvenile rats. Wistar male rats received intraperitoneal injections of MPH (2.0 mg/kg) or saline solution (controls), once a day, from the 15th to the 45th day of age. Results showed that MPH altered amino acid profile in the hippocampus, decreasing glutamine levels. Glutamate uptake and Na+,K+-ATPase activity were decreased after chronic MPH exposure in the hippocampus of rats. No changes were observed in the immunocontents of glutamate transporters (GLAST and GLT-1), and catalytic subunits of Na+,K+-ATPase (α1, α2, and α3), as well as redox status. Moreover, MPH provoked a decrease in ATP levels in the hippocampus of chronically exposed rats, while citrate synthase, succinate dehydrogenase, respiratory chain complexes activities (II, II–III, and IV), as well as mitochondrial mass and mitochondrial membrane potential were not altered. Taken together, our results suggest that chronic MPH exposure at early age impairs glutamate uptake and Na+,K+-ATPase activity probably by decreasing in ATP levels observed in rat hippocampus.




Similar content being viewed by others
References
Arnsten AF (2006) Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology 31:2376–2383
Biederman J (2005) Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry 57:1215–1220
Hannestad J, Gallezot JD, Planeta-Wilson B, Lin SF, Williams WA, van Dyck CH et al (2010) Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatry 68:854–860
Mohammadi MR, Mohammadzadeh S, Akhodzadeh S (2015) Memantine versus methylphenidate in children and adolescents with attention deficit hyperactivity disorder: a double-blind, randomized clinical tiral. Iranian J Psychiatry 10:106–114
Smith ME, Farah MJ (2011) Are prescription stimulants “smart pills”? The epidemiology and cognitive neuroscience of prescription stimulant use by normal healthy individuals. Psychological Bulleti 137:717–741
Zito JM, Safer DJ, dos Reis S, Gardner JF, Boles M, Lynch F (2000) Trends in the prescribing of psychotropic medications to preschoolers. JAMA 283:1025–1030
Adriani W, Leo D, Greco D, Rea M, di Porzio U, Laviola G, Perrone-Capano C (2006) Methylphenidate administration to adolescent rats determines plastic changes on reward-related behavior and striatal gene expression. Neuropsychopharmacology 31:1946–1956
Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA Jr (2002) Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci 5:13–14
Carlezon WA Jr, Mague SD, Andersen SL (2003) Enduring behavioral effects of early exposure to methylphenidate in rats. Biol Psychiatry 54:1330–1337
Carlezon WA Jr, Konradi C (2004) Understanding the neurobiological consequences of early exposure to psychotropic drugs: linking behavior with molecules. Neuropharmacology 47:47–60
Mague SD, Andersen SL, Carlezon WA Jr (2005) Early developmental exposure to methylphenidate reduces cocaine-induced potentiation of brain stimulation reward in rats. Biol Psychiatry 57:120–125
Gonçalves J, Baptista S, Silva AP (2014) Psychostimulants and brain dysfunction: a review of the relevant neurotoxic effects. Neuropharmacology 87:135–149
Motaghinejad M, Motevalian M, Shabbab B (2016) Effects of chronic treatment with methylphenidate on oxidative stress and inflammation in hippocampus of adult rats. Neurosci Lett 619:106–113
Réus GZ, Scaini G, Jeremias GC, Furlanetto CB, Morais MOS, Mello-Santos LM et al (2014) Brain apoptosis signaling pathways are regulated by methylphenidate in young and adult rats. Brain Res 1583:269–276
Sadasivan S, Pond BB, Pani AK, Qu C, Jiao Y, Smeyne RJ (2012) Methylphenidate exposure induces dopamine neuron loss and activation of microglia in the basal ganglia of mice. PLoS One 7:e33693
Lagace DC, Yee JK, Bolanos CA, Eisch AJ (2006) Juvenile administration of methylphenidate attenuates adult hippocampal neurogenesis. Biol Psychiatry 60:1121–1130
Schmitz F, Pierozan P, Rodrigues AF, Biasibetti H, Coelho DM, Mussulini BH, Pereira MS, Parisi MM et al (2015) Chronic treatment with a clinically relevant dose of methylphenidate increases glutamate levels in cerebrospinal fluid and impairs glutamatergic homeostasis in prefrontal cortex of juvenile rats. Mol Neurobiol 53:2384–2396
Marks’ Basic Medical Biochemistry 2009: a clinical approach by Michael A Lieberman, Allan D. Marks, 3rd edition
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
Segovia G, Porras A, Del Arco A et al (2001) Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev 122:1–29
Nicholls DG (2008) Oxidative stress and energy crises in neuronal dysfunction. Ann N Y Acad Sci 1147:53–60
Maragakis NJ, Rothstein JD (2001) Glutamate transporters in neurologic disease. Arch Neurol 58:365–370
Maragakis NJ, Rothstein JD (2004) Glutamate transporters: animal models to neurologic disease. Neurobiol Dis 15:461–473
Anderson CM, Swanson RA (2000) Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32:1–14
Zou J, Wang YX, Lü HZ, Lu PH, Xu XM (2010) Glutamine synthetase down-regulation reduces astrocyte protection against glutamate excitotoxicity to neurons. Neurochem Int 56:577–584
Rose EM, Koo JC, Antflick JE et al (2009) Glutamate transporter coupling to Na+,K+-ATPase. J Neurosci 29:8143–8155
Kaplan JH (2002) Biochemistry of Na+,K+-ATPase. Annu Ver Biochem 71:511–535
Kurup AR, Kurup PA (2002) Membrane Na+,K+-ATPase mediated cascade in bipolar mood disorder, major depressive disorder, and schizophrenia—relationship to hemispheric dominance. Int J Neurosci 112:965–982
Wyse ATS, Streck EL, Worm P, Wajner A, Ritter F, Netto CA (2000) Preconditioning prevents the inhibition of Na+,K+-ATPase activity after brain ischemia. Neurochem Res 25:971–975
Mobasheri A, Avila J, Cozar-Castellano I, Brownleader MD, Trevan M, Francis MJ et al (2000) Na+,K+-ATPase isozyme diversity; comparative biochemistry and physiological implications of novel functional interactions. Biosci Rep 20:51–91
Fukami G, Hashimoto K, Koike K, Okamura N, Shimizu E, Iyo M (2004) Effect of antioxidant N-acetyl-cysteine on behavioral changes and neurotoxicity in rats after administration of methanphetamine. Brain Res 1016:90–95
Porrino LJ, Lucignanai G (1987) Different patterns of local brain energy metabolism associated with high and low doses of methylphenidate. Relevance to its action in hyperactive children. Biol Psychiatry 22:126–138
Fagundes AO, Rezin GT, Zanette F, Grandi E, Assis LC, Dal-Pizzol F et al (2007) Chronic administration of methylphenidate activates mitochondrial respiratory chain in brain of young rats. Int J Dev Neurosci 25:47–51
Fagundes AO, Aguiar MR, Aguiar CS, Scaini G, Sachet MU, Bernhardt NM et al (2010a) Effect of acute and chronic administration of methylphenidate on mitochondrial respiratory chain in the brain of young rats. Neurochem Res 35:1675–1680
Fagundes AO, Scaini G, Santos PM, Sachet MU, Bernhardt NM, Rezin GT et al (2010b) Inhibition of mitochondrial respiratory chain in the brain of adult rats after acute and chronic administration of methylphenidate. Neurochem Res 35:405–411
Réus GZ, Scaini G, Furlanetto CB, Morais MO, Jeremias IC, Mello Santos LM et al (2013) Methylphenidate treatment leads to abnormalities on Krebs cycle enzymes in the brain of young and adult rats. Neurotoxic Res 24:251–257
Réus GZ, Scaini G, Titus SE, Furlanetto CB, Wessler LB, Ferreira GK et al (2015) Methylphenidate increases glucose uptake in the brain of young and adult rats. Pharmacol Rep 67:1033–1040
Schmitz F, Scherer EB, da Cunha MJ, da Cunha AA, Lima DD, Delwing D et al (2012) Chronic methylphenidate administration alters antioxidant defenses and butyrylcholinesterase activity in blood of juvenile rats. Mol Cell Biochem 361:281–288
Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D et al (2000) Comparison between intraperitoneal and oral methylphenidate administration: a microdialysis and locomotor activity study. J Pharmacol Exp Ther 295:51–57
Rice D, Barone S Jr (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108:511–533
Andreazza AC, Frey BN, Valvassori SS, Zanotto C, Gomes KM, Comim CM et al (2007) DNA damage in rats after treatment with methylphenidate. Prog Neuro-Psychopharmacol Biol Psychiatry 31:1282–1288
Joseph MH, Marsden CA (1986) Amino acids and small peptides. In: Lim CF (ed) HPLC of small peptides. IRL Press, Oxford
Frizzo ME, Lara DR, Prokopiuk Ade S et al (2002) Guanosine enhances glutamate uptake in brain cortical slices at normal and excitotoxic conditions. Cell Mol Neurobiol 22:353–363
Chan KM, Delfert D, Junger KD (1986) Adirect colorimetric assay for Ca2+-stimulated ATPase activity. Anal Biochem 157:375–380
LeBel CP, Ali SF, McKee M, Bondy SC (1990) Organometal-induced increases in oxygen reactive species: the potential of 2′,7′-dichlorofluorescin diacetate as an index of neurotoxic damage. Toxicol Appl Pharmacol 104:17–24
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145
Marklund SL (1985) Pyrogallol autoxidation. In: Handbook for oxygen radical research. CRC Press, Boca Raton, pp 243–247
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Witt KA, Mark KS, Hom S, Davis TP (2003) Effects of hypoxia-reoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol 285:2820–2831
Ramirez O, Jimenez E (2000) Opposite transitions of chick brain catalytically active cytosolic creatine kinase isoenzymes during development. Int J Dev Neurosci 18:815–823
Srere PA (1969) Citrate synthase. Methods Enzymol 13:3–11
Fischer JC, Ruitenbeek W, Berden JA, Trijbels JM, Veerkamp JH, Stadhouders JM et al (1985) Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta 153:23–36
Rustin P, Chretien D, Bourgeron T, Gérard B, Rötig A, Saudubray JM et al (1994) Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 228:35–51
Keij JF, Bell-Prince C, Steinkamp JA (2000) Staining of mitochondrial membranes with 10-nonyl acridine orange, MitoFluor Green, and MitoTracker Green is affected by mitochondrial membrane potential altering drugs. Cytometry A 39:203–210
Pendergrass W, Wolf N, Poot M (2004) Efficacy of MitoTracker Green (TM) and CMXRosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytometry A 61:162–169
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:346–356
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Kuczenski R, Segal DS (2002) Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci 22:7264–7271
Carlezon WA Jr, Duman RS, Nestler EJ (2005) The many faces of CREB. Trends Neurosci 28:436–44554
Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM (2002) Neurobiology of depression. Neuron 34:13–25
Cepeda C, Levine MS (2006) Where do you think you are going? The NMDA-D1 receptor trap. Science’s Signal Transduction Knowledge Environment: STKE 2006:20–24
Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B et al (2006) Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry 60:1111–1120
Scherer EB, Matté C, Ferreira AG, Gomes KM, Comim CM, Mattos C et al (2009) Methylphenidate treatment increases Na+,K+-ATPase in the cerebrum of young and adult rats. J Neural Transm 116:1681–1687
Zakharova IO, Sokolova TV, Furaev VV, Rychkova MP, Avrova NF (2007) Effects of oxidative stress inducers, neurotoxins, and ganglioside GM1 on Na+,K+-ATPase in PC12 and brain synaptosomes. Zh Evol Biokhim Fiziol 43:148–154
Pedersen WA, Cashman NR, Mattson MP (1999) The lipid peroxidation product 4-hydroxynonenal impairs glutamate and glucose transport and choline acetyltransferase activity in NSC-19 motor neuron cells. Exp Neurol 155:1–10
Schmitz F, Scherer EB, Machado FR, da Cunha AA, Tagliari B, Netto A, Wyse AT (2012) Methylphenidate induces lipid and protein damage in prefrontal cortex, but not in cerebellum, striatum, and hippocampus of juvenile rats. Metab Brain Dis 27:605–612
Martins MR, Reinke A, Petronilho FC, Gomes KM, Dal-Pizzol F, Quevedo J (2006) Methylphenidate treatment induces oxidative stress in young rat brain. Brain Res 1078:189–197
Gomes KM, Petronilho FC, Mantovani M, Garbelotto T, Boeck CR, Dal-Pizzol F et al (2008) Antioxidant enzyme activities following acute and chronic methylphenidate treatment in young rats. Neurochem Res 33:1024–1027
Gomes KM, Inácio CG, Valvassori SS, Réus GZ, Boeck CR, Dal-Pizzol F et al (2009) Superoxide production after acute and chronic treatment with methylphenidate in young and adult rats. Neurosci Lett 465:95–98
Greenwood SM, Connolly CN (2007) Dendritic and mitochondrial changes during glutamate excitotoxicity. Neuropharmacology 53:891–898
Greenwood SM, Mizielinska SM, Frenguelli BG, Harvey J, Connoly CN (2007) Mitochondrial dysfunction and dendritic beading during neuronal toxicity. J Biol Chem 282:26235–26244
Scaini G, Fagundes AO, Rezin GT, Gomes KM, Zugno AI, Quevedo J et al (2008) Methylphenidate increases creatine kinase activity in the brain of young and adult rats. Life Sci 83:795–800
Haas H, Panula P (2003) The role of histamine and the tuberomamillary nucleus in the nervous system. Nature Reviews 4:121–130
Williams K (1994) Subunit-specific potentiation of recombinant N-methyl-D-aspartate receptors by histamine. Mol Pharmacol 46:531–541
Leurs R, Bakker RA, Timmerman H, De Esch IJP (2005) The histamine H3 receptor from gene cloning to H3 receptor drugs. Nat Med 4:107–120
LaVoie MJ, Hastings TG (1999) Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci 19:1484–1491
Page G, Peeters M, Najimi M et al (2001) Modulation of the neuronal dopamine transporter activity by the metabotropic glutamate receptor mGluR5 in rat striatal synaptosomes through phosphorylation mediated processes. J Neurochem 76:1282–1290
Spina MB, Cohen G (1989) Dopamine turnover and glutathione oxidation: implications for Parkinson disease. Proc Natl Acad Sci U S A 86:1398–1400
Adam-Vizi V (2005) Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal 7:1140–1149
Navarro A, Boveris A (2007) The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol 292:670–686
Gruno M, Peet N, Tein A et al (2008) Atrophic gastritis: deficient complex I of the respiratory chain in the mitochondria of corpus mucosal cells. J Gastroenterol 43:780–788
Sen T, Sen N, Jana S et al (2007) Depolarization and cardiolipin depletion in aged rat brain mitochondria: relationship with oxidative stress and electron transport chain activity. Neurochem Int 50:719–725
Beal MF (1992) Does impairment of energy metabolism result in excitotoxic neuronal death in neurological illnesses? Ann Neurol 31:119–130
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795
Schmitz F, Pierozan P, Rodrigues AF, Biasibetti H, Grunevald M, Petenuzzo L et al (2016) Methylphenidate causes behavioral impairments and neuron and astrocyte loss in the hippocampus of juvenile rats. Mol Neurobiol. doi:10.1007/s12035-016-9987-y
Akay AP, Kaya GÇ, Emiroglu NI, Aydin A, Monkul ES, Tasçi C et al (2006) Effects of long-term methylphenidate treatment: a pilot follow-up clinical and SPECT study. Prog Neuropsychopharmacology Biol Psychiatry 30:1219–1224
Dafny N, Yang PB (2006) The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: a review of its locomotor effects. Brain Res Bull 68:393–405
Barker GR, Warburton EC (2008) NMDA receptor plasticity in the perirhinal and prefrontal cortices is crucial for the acquisition of long-term object-in-place associative memory. J Neurosci 28:2837–2844
Bergami M, Rimondini R, Santi S, Blum R, Götz M, Canossa M (2008) Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A 105:15570–15575
Heales SJ, Bolan˜os JP, Stewart VC (1999) Nitric oxide, mitochondria and neurological disease. Biochim Biophys Acta 1410:215–228
Blass JP (2001) Brain metabolism and brain disease: is metabolic deficiency the proximate cause of Alzheimer dementia? J Neurosci Res 66:851–856
Schurr A (2002) Energy metabolism, stress hormones and neural recovery from cerebral ischemia/hypoxia. Neurochem Int 41:1–8
Land JM, Morgan-Hughes JA, Hargreaves I et al (2004) Mitochondrial disease: a historical, biochemical, and London perspective. Neurochem Res 29:483–491
Acknowledgements
This work was supported in part by grants from Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq–Brazil).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest with the contents of this article.
Rights and permissions
About this article
Cite this article
Schmitz, F., Pierozan, P., Rodrigues, A.F. et al. Methylphenidate Decreases ATP Levels and Impairs Glutamate Uptake and Na+,K+-ATPase Activity in Juvenile Rat Hippocampus. Mol Neurobiol 54, 7796–7807 (2017). https://doi.org/10.1007/s12035-016-0289-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0289-1