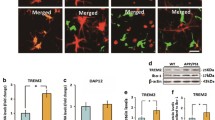
Abstract
Previously, we showed that overexpression of triggering receptor expressed on myeloid cells 2 (TREM2), a microglia-specific immune receptor, in the brain of a middle-aged (7 months old) APPswe/PS1dE9 mice could ameliorate Alzheimer’s disease (AD)-related neuropathology by enhancement of microglial amyloid-β (Aβ) phagocytosis. Since AD is an age-related neurodegenerative disorder, it is critical to assess the efficacy of TREM2 overexpression in aging animals with an advanced disease stage. In vivo, we employed a lentiviral strategy to overexpress TREM2 in the brain of aging (18 months old) APPswe/PS1dE9 mice, and observed its efficacy on AD-related neuropathology and cognitive functions. Afterwards, we directly isolated microglia from middle-aged and aging APPswe/PS1dE9 mice and determined effects of TREM2 overexpression on microglial Aβ phagocytosis and Aβ-binding receptors expression in vitro. In aging APPswe/PS1dE9 mice, TREM2 overexpression has no beneficial effect on AD-related neuropathology and spatial cognitive functions. Of note, in vitro experiments showed a significant reduction of Aβ phagocytosis in microglia from aging APPswe/PS1dE9 mice, possibly attributing to the declined expression of Aβ-binding receptors. Meanwhile, this phagocytic deficit in microglia from aging APPswe/PS1dE9 mice cannot be rescued by TREM2 overexpression. Taken together, our study shows that TREM2 overexpression fails to provide neuroprotection in aging APPswe/PS1dE9 mice, possibly attributing to deficits in microglial Aβ phagocytosis at the late-stage of disease progression. These findings indicate that TREM2-mediated protection in AD is at least partially dependent on the reservation of microglial phagocytic functions, emphasizing the importance of early therapeutic interventions for this devastating disease.






Similar content being viewed by others
References
Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C et al (2013) TREM2 variants in Alzheimer's disease. N Engl J Med 368(2):117–127. doi:10.1056/NEJMoa1211851
Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J et al (2013) Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med 368(2):107–116. doi:10.1056/NEJMoa1211103
Yu JT, Jiang T, Wang YL, Wang HF, Zhang W, Hu N, Tan L, Sun L et al (2014) Triggering receptor expressed on myeloid cells 2 variant is rare in late-onset Alzheimer's disease in Han Chinese individuals. Neurobiol Aging 35(4):937 e931–933. doi:10.1016/j.neurobiolaging.2013.10.075
Neumann H, Daly MJ (2013) Variant TREM2 as risk factor for Alzheimer's disease. N Engl J Med 368(2):182–184. doi:10.1056/NEJMe1213157
Jiang T, Yu JT, Zhu XC, Tan L (2013) TREM2 in Alzheimer's disease. Mol Neurobiol 48(1):180–185. doi:10.1007/s12035-013-8424-8
Colonna M (2003) TREMs in the immune system and beyond. Nat Rev Immunol 3(6):445–453. doi:10.1038/nri1106
Hu N, Tan MS, Yu JT, Sun L, Tan L, Wang YL, Jiang T (2014) Increased expression of TREM2 in peripheral blood of Alzheimer's disease patients. J Alzheimer's Dis: JAD 38(3):497–501. doi:10.3233/JAD-130854
Ma J, Jiang T, Tan L, Yu JT (2015) TYROBP in Alzheimer's disease. Mol Neurobiol 51(2):820–826. doi:10.1007/s12035-014-8811-9
Takahashi K, Rochford CD, Neumann H (2005) Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med 201(4):647–657. doi:10.1084/jem.20041611
Neumann H, Takahashi K (2007) Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J Neuroimmunol 184(1–2):92–99. doi:10.1016/j.jneuroim.2006.11.032
Frank S, Burbach GJ, Bonin M, Walter M, Streit W, Bechmann I, Deller T (2008) TREM2 is upregulated in amyloid plaque-associated microglia in aged APP23 transgenic mice. Glia 56(13):1438–1447. doi:10.1002/glia.20710
Jiang T, Yu JT, Zhu XC, Tan MS, Gu LZ, Zhang YD, Tan L (2014) Triggering receptor expressed on myeloid cells 2 knockdown exacerbates aging-related neuroinflammation and cognitive deficiency in senescence-accelerated mouse prone 8 mice. Neurobiol Aging 35(6):1243–1251. doi:10.1016/j.neurobiolaging.2013.11.026
Jiang T, Tan L, Zhu XC, Zhang QQ, Cao L, Tan MS, Gu LZ, Wang HF et al (2014) Upregulation of TREM2 ameliorates neuropathology and rescues spatial cognitive impairment in a transgenic mouse model of Alzheimer's disease. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol 39(13):2949–2962. doi:10.1038/npp.2014.164
Herrup K (2010) Reimagining Alzheimer's disease—an age-based hypothesis. J Neurosci: Off J Soc Neurosci 30(50):16755–16762. doi:10.1523/JNEUROSCI.4521-10.2010
Jiang T, Yu JT, Tian Y, Tan L (2013) Epidemiology and etiology of Alzheimer's disease: from genetic to non-genetic factors. Curr Alzheimer Res 10(8):852–867
Harman D (2002) Alzheimer's disease: role of aging in pathogenesis. Ann N Y Acad Sci 959:384–395, discussion 463–385
Jiang T, Yu JT, Zhu XC, Tan MS, Wang HF, Cao L, Zhang QQ, Shi JQ et al (2014) Temsirolimus promotes autophagic clearance of amyloid-beta and provides protective effects in cellular and animal models of Alzheimer's disease. Pharmacol Res: Off J Ital Pharmacol Soc 81:54–63. doi:10.1016/j.phrs.2014.02.008
Hooijmans CR, de Vries R, Leenaars M, Curfs J, Ritskes-Hoitinga M (2011) Improving planning, design, reporting and scientific quality of animal experiments by using the Gold Standard Publication Checklist, in addition to the ARRIVE guidelines. Br J Pharmacol 162(6):1259–1260. doi:10.1111/j.1476-5381.2010.01128.x
McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL (2010) Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol 160(7):1573–1576. doi:10.1111/j.1476-5381.2010.00873.x
Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010) Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160(7):1577–1579. doi:10.1111/j.1476-5381.2010.00872.x
Dodart JC, Marr RA, Koistinaho M, Gregersen BM, Malkani S, Verma IM, Paul SM (2005) Gene delivery of human apolipoprotein E alters brain Abeta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A 102(4):1211–1216. doi:10.1073/pnas.0409072102
Tan MS, Yu JT, Jiang T, Zhu XC, Guan HS, Tan L (2014) IL12/23 p40 inhibition ameliorates Alzheimer's disease-associated neuropathology and spatial memory in SAMP8 mice. J Alzheimer's Dis JAD 38(3):633–646. doi:10.3233/JAD-131148
Moreno-Gonzalez I, Estrada LD, Sanchez-Mejias E, Soto C (2013) Smoking exacerbates amyloid pathology in a mouse model of Alzheimer's disease. Nat Commun 4:1495. doi:10.1038/ncomms2494
Li MM, Jiang T, Sun Z, Zhang Q, Tan CC, Yu JT, Tan L (2014) Genome-wide microRNA expression profiles in hippocampus of rats with chronic temporal lobe epilepsy. Sci Rep 4:4734. doi:10.1038/srep04734
Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, Zhang QQ, Gao L et al (2014) Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol 171(13):3146–3157. doi:10.1111/bph.12655
Moussaud S, Draheim HJ (2010) A new method to isolate microglia from adult mice and culture them for an extended period of time. J Neurosci Methods 187(2):243–253. doi:10.1016/j.jneumeth.2010.01.017
Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, Hooli B, Choi SH et al (2013) Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 78(4):631–643. doi:10.1016/j.neuron.2013.04.014
Mosher KI, Wyss-Coray T (2014) Microglial dysfunction in brain aging and Alzheimer's disease. Biochem Pharmacol 88(4):594–604. doi:10.1016/j.bcp.2014.01.008
Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ (2012) Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging 33(1):195 e191–112. doi:10.1016/j.neurobiolaging.2010.05.008
Orre M, Kamphuis W, Osborn LM, Jansen AH, Kooijman L, Bossers K, Hol EM (2014) Isolation of glia from Alzheimer's mice reveals inflammation and dysfunction. Neurobiol Aging 35(12):2746–2760. doi:10.1016/j.neurobiolaging.2014.06.004
Hickman SE, Allison EK, El Khoury J (2008) Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. J Neurosci: Off J Soc Neurosci 28(33):8354–8360. doi:10.1523/JNEUROSCI.0616-08.2008
Kleinberger G, Yamanishi Y, Suarez-Calvet M, Czirr E, Lohmann E, Cuyvers E, Struyfs H, Pettkus N (2014) Sci Transl Med 6(243):243ra286. doi:10.1126/scitranslmed.3009093
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest. This work was supported by National Natural Science Foundation of China to T.J. (81501092), J.T.Y. (81471309), L.T. (81571245), and J.Q.S. (81500916); Natural Science Foundation of Jiangsu Province to T.J. (BK20150091) and Y.D.Z. (BK20151084); China Postdoctoral Science Foundation to T.J. (2015M580448); Qingdao Key Health Discipline Development Fund; Qingdao Outstanding Health Professional Development Fund; and Shandong Provincial Collaborative Innovation Center for Neurodegenerative Disorders.
Additional information
Teng Jiang and Yu Wan contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Overexpression of TREM2 in brain of aging APPswe/PS1dE9 mice using a lentiviral strategy. a, b The mRNA levels of Trem2 and Tyrobp in cerebral cortex were detected by qRT-PCR at 2 months after lentiviral particle injection. It should be noted that the mRNA level of Tyrobp in cerebral cortex was not affected by TREM2 overexpression. Data were normalized to the levels of Gapdh mRNA. c, d The mRNA levels of Trem2 and Tyrobp in hippocampus were detected by qRT-PCR at 2 months after lentiviral particle injection. It should be noted that the mRNA level of Tyrobp in hippocampus was not affected by TREM2 overexpression. Data were normalized to the levels of Gapdh mRNA. All data were analyzed by one-way ANOVA followed by Tukey’s post hoc test. Columns represent mean ± s.d. (n = 6 per group). *P < 0.05 (GIF 466 kb)
Fig. S2
Overexpression of TREM2 expression in primary microglia isolated from middle-aged and aging APPswe/PS1dE9 mice using a lentiviral strategy. The mRNA levels of Trem2 and Tyrobp in adult microglia were detected by qRT-PCR at 72 h after transfection. It should be noted that the mRNA level of Tyrobp was not affected by TREM2 overexpression. Data were normalized to the levels of Gapdh mRNA. All data were analyzed by one-way ANOVA followed by Tukey’s post hoc test. Columns represent mean ± s.d. *P < 0.05 (GIF 293 kb)
Table S1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Jiang, T., Wan, Y., Zhang, YD. et al. TREM2 Overexpression has No Improvement on Neuropathology and Cognitive Impairment in Aging APPswe/PS1dE9 Mice. Mol Neurobiol 54, 855–865 (2017). https://doi.org/10.1007/s12035-016-9704-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9704-x