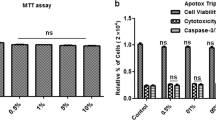
Abstract
Well-established studies have shown an elevated level of reactive oxygen species (ROS) that induces oxidative stress in the Alzheimer’s disease (AD) patient’s brain and an animal model of AD. Herein, we investigated the underlying anti-oxidant neuroprotective mechanism of natural dietary supplementation of anthocyanins extracted from Korean black beans in the amyloid precursor protein/presenilin-1 (APP/PS1) mouse model of AD. Both in vivo (APP/PS1 mice) and in vitro (mouse hippocampal HT22 cells) results demonstrated that anthocyanins regulate the phosphorylated-phosphatidylinositol 3-kinase-Akt-glycogen synthase kinase 3 beta (p-PI3K/Akt/GSK3β) pathways and consequently attenuate amyloid beta oligomer (AβO)-induced elevations in ROS level and oxidative stress via stimulating the master endogenous anti-oxidant system of nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (Nrf2/HO-1) pathways and prevent apoptosis and neurodegeneration by suppressing the apoptotic and neurodegenerative markers such as activation of caspase-3 and PARP-1 expression as well as the TUNEL and Fluoro-Jade B-positive neuronal cells in the APP/PS1 mice. In vitro ApoTox-Glo™ Triplex assay results also showed that anthocyanins act as a potent anti-oxidant neuroprotective agent and reduce AβO-induced neurotoxicity in the HT22 cells via PI3K/Akt/Nrf2 signaling. Importantly, anthocyanins improve memory-related pre- and postsynaptic protein markers and memory functions in the APP/PS1 mice. In conclusion, our data suggested that consumption and supplementation of natural-derived anti-oxidant neuroprotective agent such as anthocyanins may be beneficial and suggest new dietary-supplement strategies for intervention in and prevention of progressive neurodegenerative diseases, such as AD.






Similar content being viewed by others
References
Roychaudhuri R, Yang M, Hoshi MM, Teplow DB (2009) Amyloid β-protein assembly and Alzheimer disease. J Biol Chem 284(8):4749–4753. https://doi.org/10.1074/jbc.R800036200
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297(5580):353–356. https://doi.org/10.1126/science.1072994
De Felice FG, Velasco TP, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Kleivin WL (2007) Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem 282(15):11590–11601. https://doi.org/10.1074/jbc.M607483200
Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I et al (1998) Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A 282(11):33305–33312
Perluigi M, Sultana R, Cenini G, Di Domenico F, Memo M, Pierce WM, Coccia R, Butterfield DA (2009) Redox proteomics identification of 4-hydroxynonenal-modified brain proteins in Alzheimer’s disease: role of lipid peroxidation in Alzheimer’s disease pathogenesis. Proteomics Clin Appl 3(6):682–693. https://doi.org/10.1002/prca.200800161
Niedzielska E, Smaga I, Gawlik M, Moniczewski A, Stankowicz P, Pera J, Filip M (2016) Oxidative stress in neurodegenerative diseases. Mol Neurobiol 53(6):4094–4125. https://doi.org/10.1007/s12035-015-9337-5
Singh AK, Kashyap MP, Tripathi VK, Singh S, Garg G, Rizvi SI (2016) Neuroprotection through rapamycin-induced activation of autophagy and PI3K/Akt1/mTOR/CREB signaling against amyloid-β-induced oxidative stress, synaptic/neurotransmission dysfunction, and neurodegeneration in adult rats. Mol Neurobiol. https://doi.org/10.1007/S12035-016-0129-3
Luo J (2009) GSK3β in ethanol neurotoxicity. Mol Neurobiol 40(2):108–121. https://doi.org/10.1007/s12035-8075-y
Nakaso K, Yano H, Fukuhara Y, Takeshima T, Wada- Isoe K, Nakashima K (2003) PI3K is a key molecule in the Nrf2- mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett 546(2–3):181–184
Wang L, Chen Y, Sternberg P, Cai J (2008) Essential roles of the PI3 kinase/Akt pathway in regulating Nrf2-dependent antioxidant functions in the RPE. Invest Ophthalmol Vis Sci 49(4):1671–1678. https://doi.org/10.1167/iovs.07-1099
Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, DeGalarreta CM, Cuadrado A (2004) Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem 279(10):8919–8929. https://doi.org/10.1074/jbc.M309660200
Surh YJ, Kundu JK, Na HK (2008) Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med 74(13):1526–1539. https://doi.org/10.1055/s-0028-1088302
Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI (2011) Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 8(1):59–71. https://doi.org/10.1016/j.stem.2010.11.028
Erdogdu O, Nathanson D, Sjoholm A, Nyström T, Zhang Q (2010) Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Aktdependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol 325(1–2):26–35. https://doi.org/10.1016/j.mce.2010.04.022
Ali T, Kim MO (2015) Melatonin ameliorates amyloid beta-induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI3/Akt/GSK3β pathway in the mouse hippocampus. J Pineal Res 59(1):47–59. https://doi.org/10.1111/jpi.12238
Mhilaj E, Catino S, Miceli FM, Santangelo R, Trabace L, Cuomo V, Mancuso C (2017) Ferulic acid improves cognitive skills through the activation of the heme oxygenase system in the rat. Mol Neurobiol. https://doi.org/10.1007/s12035-017-0381-1
Zou Y, Hong B, Fan L, Zhou L, Liu Y, Wu Q, Zhang X, Dong M (2013) Protective effect of puerarin against beta-amyloid induced oxidative stress in neuronal cultures from rat hippocampus: involvement of the GSK-3β/Nrf2 signaling pathway. Free Radic Res 47(1):55–63. https://doi.org/10.3109/10715762.2012.743518
Kanninen K, Malm TM, Jyrkkänen HK, Goldsteins G, Keksa-Goldsteine V, Tanila H, Yamamoto M, Yia-Herttuala S et al (2008) Nuclear factor erythroid 2-related factor 2 protects against beta amyloid. Mol Cell Neurosci 39(3):302–313. https://doi.org/10.1016/j.mcn.2008.07.010
Kanninen K, Heikkinen R, Malm T, Rolova T, Kuhmonen S, Leinonen H, Yia-Herttuala S, Tanila H et al (2009) Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 106(38):16505–16510. https://doi.org/10.1073/pnas.0908397106
XL B, Rao PPN, Wang YJ (2016) Anti-amyloid aggregation activity of natural compounds: Implications for Alzheimer’s drug discovery. Mol Neurobiol 53(6):3565–3575. https://doi.org/10.1007/s12035-015-9301-4
Virman A, Pinto L, Binienda Z, Ali S (2013) Food, nutrigenomics, and neurodegeneration-neuroprotection by what you eat! Mol Neurobiol 48(2):353–362. https://doi.org/10.1007/s12035-013-8498-3
Shih PH, Yeh CT, Yen GC (2007) Anthocyanins induce the activation of phase ii enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J Agric Food Chem 55(23):9427–9435. https://doi.org/10.1021/jf071933i
Lan X, Wang W, Li Q, Wang J (2016) The natural flavonoid pinocembrin: molecular targets and potential therapeutic applications. Mol Neurobiol 53(3):1794–1801. https://doi.org/10.1007/s12035-015-9125-2
Schaffer S, Asseburg H, Kuntz S, Muller WE, Eckert GP (2012) Effects of polyphenols on brain ageing and Alzheimer’s disease: focus on mitochondria. Mol Neurobiol 46(1):161–178. https://doi.org/10.1007/s12035-012-8282-9
Lakey-Beitia J, Berrocal R, Rao KS, Durant AA (2015) Polyphenols as therapeutic molecules in Alzheimer’s disease through modulating amyloid pathways. Mol Neurobiol 51(2):466–479. https://doi.org/10.1007/s12035-014-8722-9
Ahmad A, Ali T, Park HY, Badshah H, Rehman SU, Kim MO (2016) Neuroprotective effect of fisetin against amyloid beta-induced cognitive/synaptic dysfunction, neuroinflammation and neurodegeneration in adult mice. Mol Neurobiol 54(3):2269–2285. https://doi.org/10.1007/s12035-016-9795-4
Shah SA, Yoon GH, Kim MO (2015) Protection of the developing brain with anthocyanins against ethanol-induced oxidative stress and neurodegeneration. Mol Neurobiol 51(3):1278–1291. https://doi.org/10.1007/s12035-014-8805-7
Rehman SU, Shah SA, Ali T, Chung JI, Kim MO (2016) Anthocyanins reversed D-galactose-induced oxidative stress and neuroinflammation mediated cognitive impairment in adult rats. Mol Neurobiol 54(1):255–271. https://doi.org/10.1007/s12035-015-9604-5
Ullah I, Park HY, Kim MO (2013) Anthocyanins protect against kainic acid-induced excitotoxicity and apoptosis via ROS-activated AMPK pathway in hippocampal neurons. CNS Neurosci Ther 20(4):327–338. https://doi.org/10.1111/cns.12218.
Shah SA, Ullah I, Lee HY, Kim MO (2013) Anthocyanins protect against ethanol-induced neuronal apoptosis via GABAB1 receptors intracellular signaling in prenatal rat hippocampal neurons. Mol Neurobiol 48(1):257–269. https://doi.org/10.1007/s12035-013-8458-y
Badshah H, Ali T, Ahmad A, Kim MJ, Abid NB, Shah SA, Yoon GH, Lee HY (2015) Co-treatment with anthocyanins and vitamin C ameliorates ethanol- induced neurodegeneration via modulation of GABAB receptor signaling in the adult rat brain. CNS Neurol Disord Drug Targets 14(6):791–803
Ye J, Meng X, Yan C, Wang C (2010) Effects of purple sweet potato anthocyanins on β-amyloid-mediated PC-12 cells death by inhibition of oxidative stress. Neurochem Res 35 (3):357–365. https://doi.org/10.1007/s11064-009-0063-0
Kang TH, Hur JY, Kim HB, Ryu JH, Kim SY (2006) Neuroprotective effects of the cyanidin-3-O-beta-d-glucopyranoside isolated from mulberry fruit against cerebral ischemia. Neurosci Lett 391(3):122–126. https://doi.org/10.1016/j.neulet.2005.08.053
Catarina R, Vauzour D, Rattray M, Waffo-Teguo P, Merillon JM, Butler LT, Williams CM, Spencer JP (2012) Dietary levels of pure flavonoids improve spatial memory performance and increase hippocampal brain-derived neurotrophic factor. PLoS One 8:e63535. https://doi.org/10.1371/journal.pone.0063535
Carvalho FB, Gutierres JM, Bueno A, Agostinho P, Zago AM, Vieira J, Fruhauf P, Cechella JL et al (2017) Anthocyanins control neuroinflammation and consequent memory dysfunction in mice exposed to lipopolysaccharide. Mol Neurobiol 54(5):3350–3367. https://doi.org/10.1007/s12035-016-9900-8
Badshah H, Kim TH, Kim MO (2015) Protective effects of anthocyanins against amyloid beta-induced neurotoxicity in vivo and in vitro. Neurochem Int 80:51–59. https://doi.org/10.1016/j.neuint.2014.10.009
Ali T, Kim MJ, Rehman SU, Ahmad A, Kim MO (2016) Anthocyanin-loaded PEG-gold nanoparticles enhanced the neuroprotection of anthocyanins in an Aβ1–42 mouse model of Alzheimer’s disease. Mol Neurobiol. https://doi.org/10.1007/s12035-016-0136-4
Ali T, Badshah H, Kim T, Kim MO (2015) Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF-KB/JNK signaling pathway in aging mouse model. J Pineal Res 58(1):71–85. https://doi.org/10.1111/jpi.12194
Ali T, Yoon GH, Shah SA, Lee HY, Kim MO (2015) Osmotin attenuates amyloid beta-induced memory impairment, tau phosphorylation and neurodegeneration in the mouse hippocampus. Sci Rep 5:11708. https://doi.org/10.1038/srep11708
Li L, Li W, Jung SW, Lee YW, Kim YH (2011) Protective effects of decursin and decursinol angelate against amyloid β-protein-induced oxidative stress in the PC12 cell line: The role of Nrf2 and antioxidant enzymes. Biosci Biotechnol Biochem 75(3):434–442
Zhou Y, Xie N, Li L, Zou Y, Zhang X, Dong M (2014) Puerarin alleviates cognitive impairment and oxidative stress in APP/PS1 transgenic mice. Int J Neuropsychopharmacol 17(4):635–644. https://doi.org/10.1017/s146114571300148x
Hamilton A, Holscher C (2012) The effect of ageing on neurogenesis and oxidative stress in the APPswe/PS1deltaE9 mouse model of Alzheimer’s disease. Brain Res 1449:83–93. https://doi.org/10.1016/j.brainres.2012.02.015
Yan J, Lai B, Xu A, Liu Y, Li X, Zhao Y, Li W, Ji M et al (2015) Maged1 co-interacting with CREB through a hexapeptide repeat domain regulates learning and memory in mice. Mol Neurobiol 51(1):8–18. https://doi.org/10.1007/s12035-014-8677-x
Gao H, Yan P, Zhang S, Huang H, Huang F, Sun T, Deng Q, Huang Q et al (2016) Long-term dietary alpha-linolenic acid supplement alleviates cognitive impairment correlate with activating hippocampal CREB signaling in natural aging rats. Mol Neurobiol 53(7):4772–4786. https://doi.org/10.1007/s12035-015-9393-x
Sultana R, Butterfield DA (2013) Oxidative modification of brain proteins in Alzheimer’s disease: perspective on future studies based on results of redox proteomics studies. J Alzheimers Dis 33(1):243–251. https://doi.org/10.3233/JAD-2012-129018
Wan L, Nie G, Zhang J, Luo Y, Zhang P, Zhang Z, Zhao B (2011) β-Amyloid peptide increases levels of iron content and oxidative stress in human cell and Caenorhabditis elegans models of Alzheimer disease. Free Radic Biol Med 50(1):122–129. https://doi.org/10.1016/j.freeradbiomed.2010.10.707
Lovell M, Markesbery W (2007) Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer’s disease. Nucleic Acids Res 35(22):7497–7504. https://doi.org/10.1093/nar/gkm821
Lee JY, Choi SI, Park HM, Lee YM, Song KJ, Kim YH, Kim KH, Hwang DY et al (2011) 4-O-Methylhonokiol attenuates memory impairment in presenilin 2 mutant mice through reduction of oxidative damage and inactivation of astrocytes and the ERK pathway. Free Radic Biol Med 50(1):66–77. https://doi.org/10.1016/j.freeradbiomed.2010.10.698
Ding Y, Chen M, Wang M, Li Y, Wen A (2015) Posttreatment with 11-Keto-β-boswellic acid ameliorates cerebral ischemia–reperfusion injury: Nrf2/HO-1 pathway as a potential mechanism. Mol Neurobiol 52(3):1430–1439. https://doi.org/10.1007/S12035-014-8929-9
Jiang S, Deng C, Lv J, Fan C, Hu W, Di S, Yan X, Ma Z et al (2017) Nrf2 weaves an elaborate network of neuroprotection against stroke. Mol Neurobiol 54(2):1440–1455. https://doi.org/10.1007/s12035-016-9707-7
Zhang R, Xu M, Wang Y, Xie F, Zhang G, Qin X (2016) Nrf2-a promising therapeutic target for defensing against oxidative stress in stroke. Mol Neurobiol. https://doi.org/10.1007/s12035-016-0111-0
Freitas AE, Egea J, Buendia I, Navarro E, Rada P, Cuadrado A, Rodrigues AL, Lopez MG (2015) Agmatine induces Nrf2 and protects against corticosterone effects in hippocampal neuronal cell line. Mol Neurobiol 51:1504–1519. https://doi.org/10.1007/s12035-014-8827-1
Zhang H, Liu YY, Jiang Q, Li K, Zhao Y (2014) Salvianolic acid a protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling. Free Radic Biol Med 69:219–228. https://doi.org/10.1016/j.freeradbiomed.2014.01.025
Kamalvand G, Pinard G, Ali-Khan Z (2003) Heme-oxygenase-1 response, a marker of oxidative stress, in a mouse model of AA amyloidosis. Amyloid 10(3):151–159
Godoy JA, Lindsay CB, Quintanilla RA, Carvajal FJ, Cerpa W, Inestrosa NC (2016) Quercetin exerts differential neuroprotective effects against H2O2 and Aβ aggregates in hippocampal neurons: the role of mitochondria. Mol Neurobiol. https://doi.org/10.1007/s12035-016-0203-x
Rojo AI, Rada P, Egea J, Rosa AO, Lopez MG, Cuadrado A (2008) Functional interference between glycogen synthase kinase-3 beta and the transcription factor Nrf2 in protection against kainate-induced hippocampal cell death. Mol Cell Neurosci 39(1):125–132. https://doi.org/10.1016/j.mcn.2008.06.007
Rojo AI, Sagarra MR, Cuadrado A (2008) GSK-3beta downregulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. J Neurochem 105(1):192–202. https://doi.org/10.1111/j.1471-4159.2007.05124.x
Schafer M, Goodenough S, Moosmann B, Behl C (2004) Inhibition of glycogen synthase kinase 3 beta is involved in the resistance to oxidative stress in neuronal HT22 cells. Brain Res 1005(1–2):84–89. https://doi.org/10.1016/j.brainres.2004.01.037
Rada P, Al R, Evrard-Todeschi N, Innamorato NG, Cotte A, Jaworski T, Tobon-Velasco JC, Devijver H et al (2012) Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/β-TrCP axis. Mol Cell Biol 32(17):3486–3499. https://doi.org/10.1128/MCB.00180-12
HC S, Ma CT, BC Y, Chien YC, Tsai CC, Huang WC, Lin CF, Chuang YH et al (2012) Glycogen synthase kinase-3β regulates anti-inflammatory property of fluoxetine. Int Immunopharmacol 14(2):150–156. https://doi.org/10.1016/j.intimp.2012.06.015
Kitagishi Y, Nakanishi A, Ogura Y, Matsuda S (2014) Dietary regulation of PI3K/AKT/GSK-3β pathway in Alzheimer’s disease. Alzheimers Res Ther 6(3). https://doi.org/10.1186/alzrt265
Chen HH, Chen YT, Huang YW, Tsai HJ, Kuo CC (2012) 4-Ketopinoresinol, a novel naturally occurring ARE activator, induces the Nrf2/HO-1 axis and protects against oxidative stress-induced cell injury via activation of PI3K/AKT signaling. Free Radic Biol Med 52(6):1054–1066. https://doi.org/10.1016/j.freeradbiomed.2011.12.012
Lou H, Jing X, Wei X, Shi H, Ren D, Zhang X (2014) Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the Nrf2/ARE signaling pathway. Neuropharmacology 79:380–388. https://doi.org/10.1016/j.neuropharm.2013.11.026
Xu J, Wang H, Ding K, Zhang L, Wang C, Li T, Wei W, Lu X (2014) Luteolin provides neuroprotection in models of traumatic brain injury via the Nrf2–ARE pathway. Free Radic Biol Med 71:186–195. https://doi.org/10.1016/j.freeradbiomed.2014.03.009
Zhao B (2005) Natural antioxidants for neurodegenerative diseases. Mol Neurobiol 31(1–3):283–293. https://doi.org/10.1385/MN:31:1-3:283
Prakash D, Sudhandiran G (2015) Dietary flavonoid fisetin regulates aluminium chloride induced neuronal apoptosis in cortex and hippocampus of mice brain. J Nutr Biochem 26(12):1527–1539. https://doi.org/10.1016/j.jnutbio.2015.07.017.
Gopinath K, Prakash D, Sudhandiran G (2011) Neuroprotective effect of naringin, a dietary flavonoid against 3-nitropropionic acid-inducedneuronal apoptosis. Neurochem Int 59(7):1066–1073. https://doi.org/10.1016/j.neuint.2011.08.022
Lan X, Han X, Li Q, Wang J (2016) (−)-Epicatechin, a natural flavonoid compound, protects astrocytes against hemoglobin toxicity via Nrf2 and AP-1 signaling pathways. Mol Neurobiol. https://doi.org/10.1007/s12035-016-027-y
Khan MS, Ali T, Kim MW, Jo MH, Jo MG, Badshah H, Kim MO (2016) Anthocyanins protect against LPS-induced oxidative stress-mediated neuroinflammation and neurodegeneration in the adult mouse cortex. Neurochem Int 100:1–10. https://doi.org/10.1016/j.neuint.2016.08.005.
Nistico R, Pignatelli M, Piccinin S, Mercuri NB, Collingridge G (2012) Targeting synaptic dysfunction in Alzheimer’s disease therapy. Mol Neurobiol 46(3):572–587. https://doi.org/10.1007/s12035-012-8324-3
Proctor DT, Coulson EJ, Dodd PR (2010) Reduction in post-synaptic scaffolding PSD-95 and SAP-102 protein in the Alzheimer inferior temporal cortex is correlated with disease pathology. J Alzheimers Dis 21(3):795–811. https://doi.org/10.3233/JAD-2010-100090
Restivo L, Tafi E, Ammassari-Teule M, Marie H (2009) Viral mediated expression of a constitutively active form of CREB in hippocampal neurons increases memory. Hippocampus 19(3):228–234. https://doi.org/10.1002/hipo.20527
Prakash D, Gopinath K, Sudhandiran G (2013) Fisetin enhances behavioral performances and attenuates reactive gliosis and inflammation during aluminum chloride-induced neurotoxicity. NeuroMolecular Med 15(1):192–208. https://doi.org/10.1007/s12017-8210-1.
Scapagnini G, Vasto S, Abraham NG, Caruso C, Zella D, Fabio G (2011) Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol 44(2):192–201. https://doi.org/10.1007/s12035-011-8181-5
Liu M, Chen F, Sha L, Wang S, Tao L, Yao L, He M, Yao Z et al (2014) (−)-Epigallocatechin-3-gallate ameliorates learning and memory deficits by adjusting the balance of TrkA/p75NTR signaling in APP/PS1 transgenic mice. Mol Neurobiol 49(3):1350–1363. https://doi.org/10.1007/s12035-013-8608-2
Cui J, Othishankar B, He P, Staufenbiel M, Shen Y, Li R (2014) Amyloid precursor protein mutation disrupts reproductive experience-enhanced normal cognitive development in a mouse model of Alzheimer’s disease. Mol Neurobiol 49(1):103–112. https://doi.org/10.1007/s12035-013-8503-x
Freits AE, Egea J, Buendia I, Gomez-Rangel V, Parada E, Navarro E, Casas AI, Wojnicz A et al (2016) Agmatine, by improving neuroplasticity markers and inducing Nrf2, prevents corticosterone-induced depressive-like behavior in mice. Mol Neurobiol 53(5):3030–3045. https://doi.org/10.1007/s12035-015-9182-6
Dwivedi S, Rajasekar N, Hanif K, Nath C, Shukla R (2016) Sulforaphane ameliorates okadaic acid-induced memory impairment in rats by activating the Nrf2/HO-1 antioxidant pathway. Mol Neurobiol 53(8):5310–5323. https://doi.org/10.1007/s12035-015-9451-4
Acknowledgements
This research was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2016M3C7A1904391).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animal maintenance, treatments, behavioral studies and surgical procedures were carried out in accordance with the animal ethics committee (IACUC) guidelines issued by the Division of Applied Life Sciences, Department of Biology at Gyeongsang National University, South Korea. The experimental methods with animals were carried out in accordance with the approved guidelines (Approval ID: 125) and all experimental protocol were approved by the animal ethics committee (IACUC) of the Division of Applied Life Sciences, Department of Biology at Gyeongsang National University, South Korea.
Conflict of Interest
The authors declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Ali, T., Kim, T., Rehman, S.U. et al. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol Neurobiol 55, 6076–6093 (2018). https://doi.org/10.1007/s12035-017-0798-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0798-6