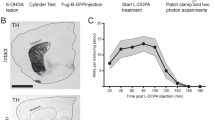
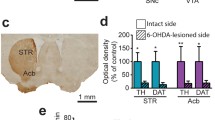
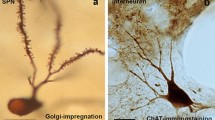
Abstract
Using bacterial artificial chromosome–double transgenic mice expressing tdTomato in D1 receptor-medium spiny neurons (MSNs) and enhanced green fluorescent protein in D2 receptor-MSNs, we have studied changes in spine density and perisomatic GABAergic boutons density in MSNs of both the D1R and D2R pathways, in an experimental model of parkinsonism (mouse injected with 6-hydroxydopamine in the medial forebrain bundle), both in the parkinsonian and dyskinetic condition induced by l-DOPA treatment. To assess changes in perisomatic GABAergic connectivity onto MSNs, we measured the number of contacts originated from parvalbumin (PV)-containing striatal “fast-spiking” interneurons (FSIs), the major component of a feed-forward inhibition mechanism that regulates spike timing in MSNs, in both cell types as well as the number of vesicular GABA transporter (VGAT) contacts. Furthermore, we determined changes in PV-immunoreactive cell density by PV immunolabeling combined with Wisteria floribunda agglutinin (WFA) labeling to detect FSI in a PV-independent manner. We also explored the differential expression of striatal activity–regulated cytoskeleton-associated protein (Arc) and c-Fos in both types of MSNs as a measure of neuronal activation. Our results confirm previous findings of major structural changes in dendritic spine density after nigrostriatal denervation, which are further modified in the dyskinetic condition. Moreover, the finding of differential modifications in perisomatic GABAergic connectivity and neuronal activation in MSNs suggests an attempt by the system to regain homeostasis after denervation and an imbalance between excitation and inhibition leading to the development of dyskinesia after exposure to l-DOPA.




Similar content being viewed by others
References
Birkmayer W, Hornykiewicz O (1961) The L-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia. Wien Klin Wochenschr 73:787–788
Cramer SC, Sur M, Dobkin BH, O’Brien C, Sanger TD, Trojanowski JQ, Rumsey JM, Hicks R et al (2011) Harnessing neuroplasticity for clinical applications. Brain 134:1591–1609. https://doi.org/10.1093/brain/awr039
Holtmaat A, Caroni P (2016) Functional and structural underpinnings of neuronal assembly formation in learning. Nat Neurosci 19:1553–1562. https://doi.org/10.1038/nn.4418
Butz M, Wörgötter F, van Ooyen A (2009) Activity-dependent structural plasticity. Brain Res Rev 60:287–305. https://doi.org/10.1016/j.brainresrev.2008.12.023
Graf ER, Zhang X, Jin S-X, Linhoff MW, Craig AM (2004) Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 119:1013–1026. https://doi.org/10.1016/j.cell.2004.11.035
Liu G (2004) Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat Neurosci 7:373–379. https://doi.org/10.1038/nn1206
Prange O, Wong TP, Gerrow K, Wang YT, el-Husseini A (2004) A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci 101:13915–13920. https://doi.org/10.1073/pnas.0405939101
Levinson JN, El-Husseini A (2005) Building excitatory and inhibitory synapses: balancing neuroligin partnerships. Neuron 48:171–174. https://doi.org/10.1016/j.neuron.2005.09.017
Kawaguchi Y (1997) Neostriatal cell subtypes and their functional roles. Neurosci Res 27:1–8
Bolam J, Hanley J, Booth P, Bevan M (2000) Synaptic organisation of the basal ganglia. J Anat 196:527–542. https://doi.org/10.1046/j.1469-7580.2000.19640527.x
Gerfen CR, Young WS (1988) Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res 460:161–167
Gerfen CR, Surmeier DJ (2011) Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34:441–466. https://doi.org/10.1146/annurev-neuro-061010-113641
Chen Y, Sabatini BL (2012) Signaling in dendritic spines and spine microdomains. Curr Opin Neurobiol 22:389–396. https://doi.org/10.1016/j.conb.2012.03.003
Suárez L, Solís O, Caramés J et al (2014) L-DOPA treatment selectively restores spine density in dopamine receptor d2-expressing projection neurons in dyskinetic mice. Biol Psychiatry 75:711–722. https://doi.org/10.1016/j.biopsych.2013.05.006
Suarez LM, Solis O, Aguado C, Lujan R, Moratalla R (2016) L-DOPA oppositely regulates synaptic strength and spine morphology in D1 and D2 striatal projection neurons in dyskinesia. Cereb Cortex 26:4253–4264. https://doi.org/10.1093/cercor/bhw263
Nishijima H, Suzuki S, Kon T, Funamizu Y, Ueno T, Haga R, Suzuki C, Arai A et al (2014) Morphologic changes of dendritic spines of striatal neurons in the levodopa-induced dyskinesia model. Mov Disord 29:336–343. https://doi.org/10.1002/mds.25826
Fieblinger T, Graves SM, Sebel LE, Alcacer C, Plotkin JL, Gertler TS, Chan CS, Heiman M et al (2014) Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced dyskinesia. Nat Commun 5:5316. https://doi.org/10.1038/ncomms6316
Zhang Y, Meredith GE, Mendoza-Elias N, Rademacher DJ, Tseng KY, Steece-Collier K (2013) Aberrant restoration of spines and their synapses in L-DOPA-induced dyskinesia: involvement of corticostriatal but not thalamostriatal synapses. J Neurosci 33:11655–11667. https://doi.org/10.1523/JNEUROSCI.0288-13.2013
Kawaguchi Y (1993) Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci 13:4908–4923
Tepper JM, Tecuapetla F, Koós T, Ibáñez-Sandoval O (2010) Heterogeneity and diversity of striatal GABAergic interneurons. Front Neuroanat 4:150. https://doi.org/10.3389/fnana.2010.00150
Mallet N, Ballion B, Le Moine C, Gonon F (2006) Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci 26:3875–3884. https://doi.org/10.1523/JNEUROSCI.4439-05.2006
Berke JD, Okatan M, Skurski J, Eichenbaum HB (2004) Oscillatory entrainment of striatal neurons in freely moving rats. Neuron 43:883–896. https://doi.org/10.1016/j.neuron.2004.08.035
Tepper JM, Koós T, Wilson CJ (2004) GABAergic microcircuits in the neostriatum. Trends Neurosci 27:662–669. https://doi.org/10.1016/j.tins.2004.08.007
Kita H, Kosaka T, Heizmann CW (1990) Parvalbumin-immunoreactive neurons in the rat neostriatum: a light and electron microscopic study. Brain Res 536:1–15
Bennett B, Bolam J (1994) Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience 62:707–719
Gittis AH, Nelson AB, Thwin MT, Palop JJ, Kreitzer AC (2010) Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J Neurosci 30:2223–2234. https://doi.org/10.1523/JNEUROSCI.4870-09.2010
Gittis AH, Hang GB, LaDow ES et al (2011) Rapid target-specific remodeling of fast-spiking inhibitory circuits after loss of dopamine. Neuron 71:858–868. https://doi.org/10.1016/j.neuron.2011.06.035
Salin P, López IP, Kachidian P, Barroso-Chinea P, Rico AJ, Gómez-Bautista V, Coulon P, Kerkerian-le Goff L et al (2009) Changes to interneuron-driven striatal microcircuits in a rat model of Parkinson’s disease. Neurobiol Dis 34:545–552. https://doi.org/10.1016/j.nbd.2009.03.006
Shuen J, Chen M, Gloss B, Calakos N (2008) Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. J Neurosci 28:2681–2685. https://doi.org/10.1523/JNEUROSCI.5492-07.2008
Escande MV, Taravini IRE, Zold CL, Belforte JE, Murer MG (2016) Loss of homeostasis in the direct pathway in a mouse model of asymptomatic parkinson’s disease. J Neurosci 36:5686–5698. https://doi.org/10.1523/JNEUROSCI.0492-15.2016
Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates, 2nd edn. Academic Press, San Diego
Cenci MA, Lundblad M (2007) Ratings of L-DOPA-induced dyskinesia in the unilateral 6-OHDA lesion model of Parkinson’s disease in rats and mice. Curr Protoc Neurosci Chapter 9:Unit 9.25. https://doi.org/10.1002/0471142301.ns0925s41
Francardo V, Recchia A, Popovic N, Andersson D, Nissbrandt H, Cenci MA (2011) Impact of the lesion procedure on the profiles of motor impairment and molecular responsiveness to L-DOPA in the 6-hydroxydopamine mouse model of Parkinson’s disease. Neurobiol Dis 42:327–340. https://doi.org/10.1016/j.nbd.2011.01.024
Espadas I, Darmopil S, Vergaño-Vera E, Ortiz O, Oliva I, Vicario-Abejón C, Martín ED, Moratalla R (2012) L-DOPA-induced increase in TH-immunoreactive striatal neurons in parkinsonian mice: insights into regulation and function. Neurobiol Dis 48:271–281. https://doi.org/10.1016/j.nbd.2012.07.012
Larramendy C, Taravini IRE, Saborido MD, Ferrario JE, Murer MG, Gershanik OS (2008) Cabergoline and pramipexole fail to modify already established dyskinesias in an animal model of parkinsonism. Behav Brain Res 194:44–51. https://doi.org/10.1016/j.bbr.2008.06.021
Lundblad M, Picconi B, Lindgren H, Cenci MA (2004) A model of L-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis 16:110–123. https://doi.org/10.1016/j.nbd.2004.01.007
Ruiz-Dediego I, Mellstrom B, Vallejo M et al (2015) Activation of DREAM (downstream regulatory element antagonistic modulator), a calcium-binding protein, reduces L-DOPA-induced dyskinesias in mice. Biol Psychiatry 77:95–105. https://doi.org/10.1016/j.biopsych.2014.03.023
Solís O, Garcia-Montes JR, González-Granillo A, Xu M, Moratalla R (2017) Dopamine D3 receptor modulates L-DOPA-induced dyskinesia by targeting D1 receptor-mediated striatal signaling. Cereb Cortex 27:435–446. https://doi.org/10.1093/cercor/bhv231
Taravini IRE, Ferrario JE, Delbe J, Ginestet L, Debeir T, Courty J, Murer MG, Gershanik OS et al (2005) Immunodetection of heparin-binding growth associated molecule (pleiotrophin) in striatal interneurons. Brain Res 1066:196–200. https://doi.org/10.1016/j.brainres.2005.10.055
Taravini IRE, Chertoff M, Cafferata EG, Courty J, Murer MG, Pitossi FJ, Gershanik OS (2011) Pleiotrophin over-expression provides trophic support to dopaminergic neurons in parkinsonian rats. Mol Neurodegener 6:40. https://doi.org/10.1186/1750-1326-6-40
Elston GN, Benavides-Piccione R, DeFelipe J (2001) The pyramidal cell in cognition: a comparative study in human and monkey. J Neurosci 21:RC163
Enwright JF, Sanapala S, Foglio A et al (2016) Reduced labeling of parvalbumin neurons and perineuronal nets in the dorsolateral prefrontal cortex of subjects with schizophrenia. Neuropsychopharmacology 41:2206–2214. https://doi.org/10.1038/npp.2016.24
Braz BY, Galiñanes GL, Taravini IR et al (2015) Altered corticostriatal connectivity and exploration/exploitation imbalance emerge as intermediate phenotypes for a neonatal dopamine dysfunction. Neuropsychopharmacology 40:1–12. https://doi.org/10.1038/npp.2015.104
Sholl DA (1953) Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87:387–406
Gagnon D, Petryszyn S, Sanchez MG, Bories C, Beaulieu JM, de Koninck Y, Parent A, Parent M (2017) Striatal neurons expressing D1 and D2 receptors are morphologically distinct and differently affected by dopamine denervation in mice. Sci Rep 7:9–17. https://doi.org/10.1038/srep41432
McNeill TH, Brown SA, Rafols JA, Shoulson I (1988) Atrophy of medium spiny I striatal dendrites in advanced Parkinson’s disease. Brain Res 455:148–152
Zaja-Milatovic S, Milatovic D, Schantz AM, Zhang J, Montine KS, Samii A, Deutch AY, Montine TJ (2005) Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology 64:545–547. https://doi.org/10.1212/01.WNL.0000150591.33787.A4
Gittis AH, Leventhal DK, Fensterheim B et al (2011) Selective inhibition of striatal fast-spiking interneurons causes dyskinesias. J Neurosci 31:15727–15731. https://doi.org/10.1523/JNEUROSCI.3875-11.2011
Galarraga E, Vilchis C, Tkatch T, Salgado H, Tecuapetla F, Perez-Rosello T, Perez-Garci E, Hernandez-Echeagaray E et al (2007) Somatostatinergic modulation of firing pattern and calcium-activated potassium currents in medium spiny neostriatal neurons. Neuroscience 146:537–554. https://doi.org/10.1016/j.neuroscience.2007.01.032
Kubota Y, Kawaguchi Y (2000) Dependence of GABAergic synaptic areas on the interneuron type and target size. J Neurosci 20:375–386
Straub C, Saulnier JL, Bègue A, Feng DD, Huang KW, Sabatini BL (2016) Principles of synaptic organization of GABAergic interneurons in the striatum. Neuron 92:84–92. https://doi.org/10.1016/j.neuron.2016.09.007
DiFiglia M, Aronin N (1982) Ultrastructural features of immunoreactive somatostatin neurons in the rat caudate nucleus. J Neurosci 2:1267–1274
Chuhma N, Tanaka KF, Hen R, Rayport S (2011) Functional connectome of the striatal medium spiny neuron. J Neurosci 31:1183–1192. https://doi.org/10.1523/JNEUROSCI.3833-10.2011
Wilson CJ, Groves PM (1980) Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. J Comp Neurol 194:599–615. https://doi.org/10.1002/cne.901940308
Caillard O, Moreno H, Schwaller B, Llano I, Celio MR, Marty A (2000) Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc Natl Acad Sci U S A 97:13372–13377. https://doi.org/10.1073/pnas.230362997
Orduz D, Bischop DP, Schwaller B, Schiffmann SN, Gall D (2013) Parvalbumin tunes spike-timing and efferent short-term plasticity in striatal fast spiking interneurons. J Physiol 591:3215–3232. https://doi.org/10.1113/jphysiol.2012.250795
Filice F, Vörckel KJ, Sungur AÖ, Wöhr M, Schwaller B (2016) Reduction in parvalbumin expression not loss of the parvalbumin-expressing GABA interneuron subpopulation in genetic parvalbumin and shank mouse models of autism. Mol Brain 9:10. https://doi.org/10.1186/s13041-016-0192-8
Parker JG, Marshall JD, Ahanonu B, Wu YW, Kim TH, Grewe BF, Zhang Y, Li JZ et al (2018) Diametric neural ensemble dynamics in parkinsonian and dyskinetic states. Nature 557:177–182. https://doi.org/10.1038/s41586-018-0090-6
Girasole AE, Lum MY, Nathaniel D, Bair-Marshall CJ, Guenthner CJ, Luo L, Kreitzer AC, Nelson AB (2018) A subpopulation of striatal neurons mediates levodopa-induced dyskinesia. Neuron 97:787–795.e6. https://doi.org/10.1016/j.neuron.2018.01.017
Wang JQ, Smith AJ, McGinty JF (1995) A single injection of amphetamine or methamphetamine induces dynamic alterations in c-fos, zif/268 and preprodynorphin messenger RNA expression in rat forebrain. Neuroscience 68:83–95
Kravitz AV, Freeze BS, Parker PRL et al (2010) Regulation of parkinsonian motor behaviors by optogenetic control of basal ganglia circuitry. Nature 466:622–626. https://doi.org/10.1038/nature09159.Regulation
Aubert I, Guigoni C, Håkansson K, Li Q, Dovero S, Barthe N, Bioulac BH, Gross CE et al (2005) Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol 57:17–26. https://doi.org/10.1002/ana.20296
Picconi B, Centonze D, Håkansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P (2003) Loss of bidirectional striatal synaptic plasticity in L-DOPA–induced dyskinesia. Nat Neurosci 6:501–506. https://doi.org/10.1038/nn1040
Picconi B, Paillé V, Ghiglieri V, Bagetta V, Barone I, Lindgren HS, Bernardi G, Angela Cenci M et al (2008) l-DOPA dosage is critically involved in dyskinesia via loss of synaptic depotentiation. Neurobiol Dis 29:327–335. https://doi.org/10.1016/j.nbd.2007.10.001
Andersson M, Hilbertson A, Cenci MA (1999) Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson’s disease. Neurobiol Dis 6:461–474. https://doi.org/10.1006/nbdi.1999.0259S0969-9961(99)90259-0
Feyder M, Bonito-Oliva A, Fisone G (2011) L-DOPA-induced dyskinesia and abnormal signaling in striatal medium spiny neurons: focus on dopamine D1 receptor-mediated transmission. Front Behav Neurosci 5:71. https://doi.org/10.3389/fnbeh.2011.00071
Westin JE, Vercammen L, Strome EM, Konradi C, Cenci MA (2007) Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of L-DOPA-induced dyskinesia and the role of dopamine D1 receptors. Biol Psychiatry 62:800–810. https://doi.org/10.1016/j.biopsych.2006.11.032
Sgambato-Faure V, Buggia V, Gilbert F, Lévesque D, Benabid AL, Berger F (2005) Coordinated and spatial upregulation of arc in striatonigral neurons correlates with L-dopa-induced behavioral sensitization in dyskinetic rats. J Neuropathol Exp Neurol 64:936–947. https://doi.org/10.1097/01.jnen.0000186922.42592.b7
Bastide MF, Dovero S, Charron G, Porras G, Gross CE, Fernagut PO, Bézard E (2014) Immediate-early gene expression in structures outside the basal ganglia is associated to l-DOPA-induced dyskinesia. Neurobiol Dis 62:179–192. https://doi.org/10.1016/j.nbd.2013.09.020
Steward O, Wallace CS, Lyford GL, Worley PF (1998) Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron 21:741–751
Steward O, Worley P (2002) Local synthesis of proteins at synaptic sites on dendrites: role in synaptic plasticity and memory consolidation? Neurobiol Learn Mem 78:508–527
Shen W, Flajolet M, Greengard P, Surmeier DJ (2008) Dichotomous dopaminergic control of striatal synaptic plasticity. Science 321:848–851. https://doi.org/10.1126/science.1160575
Bello EP, Casas-Cordero R, Galiñanes GL, Casey E, Belluscio MA, Rodríguez V, Noaín D, Murer MG et al (2017) Inducible ablation of dopamine D2 receptors in adult mice impairs locomotion, motor skill learning and leads to severe parkinsonism. Mol Psychiatry 22:595–604. https://doi.org/10.1038/mp.2016.105
Acknowledgements
The authors would like to thank Germán La Iacona for his technical assistance.
Funding
This work was supported by grants from the Argentine Agency for the Promotion of Science (PICT 2011-1758 and PICT 2015-3687), University of Buenos Aires (UBACyT 2014-2017 249), and Argentine National Research Council (CONICET, PIP 2013-0401) and by grants from the Spanish Ministries of Economía, Industria y Competitividad (SAF2016-78207-R) and PCIN-2015-098 and of Sanidad Servicios Sociales e Igualdad (ISCIII, CIBERNED CB06/05/0055, PNSD2016I033) and 172275 from Ramón Areces Foundation to RM. GG is a research fellow of the CONICET. LR, JEB, MGM, and IRET are members of the research career of CONICET.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All surgical procedures and experimental manipulations were performed in accordance with European Council Directive 2010/63/EU guidelines for the care of laboratory animals and the regulations for the Care and Use of Laboratory Animals of the National Institutes of Health, USA. Animal experiments were approved by our local Ethics Committee (IACUC EXP-UBA No. 0027665/2014).
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gomez, G., Escande, M.V., Suarez, L.M. et al. Changes in Dendritic Spine Density and Inhibitory Perisomatic Connectivity onto Medium Spiny Neurons in l-Dopa-Induced Dyskinesia. Mol Neurobiol 56, 6261–6275 (2019). https://doi.org/10.1007/s12035-019-1515-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1515-4