Abstract
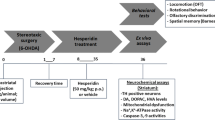
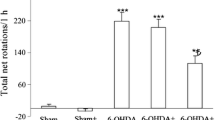
The mechanisms underlying the neuroprotective effects of hesperidin in a murine model of PD are not fully elucidated. The current study was carried out to investigate the ability of hesperidin in modulating proinflammatory cytokines, neurotrophic factors, and neuronal recovery in 6-hydroxydopamine (6-OHDA)-induced nigral dopaminergic neuronal loss. Adult male C57BL/6 mice were randomly assigned into four groups: (I) sham/vehicle, (II) sham/hesperidin, (III) 6-OHDA/vehicle, and (IV) 6-OHDA/hesperidin. Mice received a unilateral intrastriatal injection of 6-OHDA and treated with hesperidin (50 mg/kg; per oral) for 28 days. After hesperidin treatment, mice were submitted to behavioral tests and had the striatum removed for neurochemical assays. Our results demonstrated that oral treatment with hesperidin ameliorated the anxiety-related and depressive-like behaviors in 6-OHDA-lesioned mice (p < 0.05). It also attenuated the striatal levels of proinflammatory cytokines tumor necrosis factor-α, interferon-gamma, interleukin-1β, interleukin-2, and interleukin-6 and increased the levels of neurotrophic factors, including neurotrophin-3, brain-derived neurotrophic factor, and nerve growth factor in the striatum of 6-OHDA mice (p < 0.05). Hesperidin treatment was also capable to increase striatal levels of dopamine and its metabolite 3,4-dihydroxyphenylacetic acid and protects against the impairment of dopaminergic neurons in the substantia nigra pars compacta (SNpc) (p < 0.05). In conclusion, this study indicated that hesperidin exerts anxiolytic-like and antidepressant-like effect against 6-OHDA-induced neurotoxicity through the modulation of cytokine production, neurotrophic factors levels, and dopaminergic innervation in the striatum.









Similar content being viewed by others
Abbreviations
- 6-OHDA:
-
6-hydroxydopamine
- BDNF:
-
Brain-derived neurotrophic factor
- DA:
-
Dopamine
- DOPAC:
-
3,4-dihydroxyphenylacetic acid
- EPMT:
-
Elevated plus-maze test
- GDNF:
-
Glial cell-derived neurotrophic factor
- HVA:
-
Homovanillic acid
- IFN-γ:
-
Interferon-gamma
- IL-10:
-
Interleukin-10
- IL-1β:
-
Interleukin-1beta
- IL-2:
-
Interleukin-2
- IL-6:
-
Interleukin-6
- NGF:
-
Nerve growth factor
- NT-3:
-
Neurotrophin-3
- OFT:
-
Open field test
- PD:
-
Parkinson’s disease
- TH:
-
Tyrosine hydroxylase
- TNF-α:
-
Tumor necrosis factor-alpha
References
Lees AJ, Hardy J, Revesz T (2009) Parkinson’s disease. Lancet 373:2055–2066
Shulman JM, De Jager PL, Feany MB (2011) Parkinson's disease: Genetics and pathogenesis. Annu Rev Pathol 2011:193–222
Wichowicz HM, Sławek J, Derejko M, Cubała WJ (2006) Factors associated with depression in Parkinson’s disease: a cross-sectional study in a Polish population. Eur Psychiatry 21:516–520. https://doi.org/10.1016/j.eurpsy.2006.01.012
Dissanayaka NN, Sellbach A, Silburn PA, O'Sullivan JD, Marsh R, Mellick GD (2011) Factors associated with depression in Parkinson's disease. J Affect Disord 132:82–88
Kalia LV, Lang AE (2015) Parkinson's disease. Lancet 386:896–912
Craft JM, Watterson DM, Van Eldik LJ (2005) Neuroinflammation: a potential therapeutic target. Expert Opin Ther Targets 9:887–900
Moosavi F, Hosseini R, Saso L, Firuzi O (2015) Modulation of neurotrophic signaling pathways by polyphenols. Drug Des Devel Ther 10:23–42
Pramanik S, Sulistio YA, Heese K (2017) Neurotrophin signaling and stem cells—implications for neurodegenerative diseases and stem cell therapy. Mol Neurobiol 54:7401–7459
Jiang X, Ganesan P, Rengarajan T, Choi DK, Arulselvan P (2018) Cellular phenotypes as inflammatory mediators in Parkinson’s disease: Interventional targets and role of natural products. Biomed Pharmacother 106:1052–1062
Kim HD, Jeong KH, Jung UJ, Kim SR (2016) Naringin treatment induces neuroprotective effects in a mouse model of Parkinson’s disease in vivo, but not enough to restore the lesioned dopaminergic system. J Nutr Biochem 28:140–146. https://doi.org/10.1016/j.jnutbio.2015.10.013
Vivekanantham S, Shah S, Dewji R, Dewji A, Khatri C, Ologunde R (2015) Neuroinflammation in Parkinson’s disease: role in neurodegeneration and tissue repair. Int J Neurosci 125:717–725
Blandini F, Armentero MT (2012) Animal models of Parkinson’s disease. FEBS J 279:1156–1166
Santiago RM, Barbieiro J, Lima MM et al (2010) Depressive-like behaviors alterations induced by intranigral MPTP, 6-OHDA, LPS and rotenone models of Parkinson's disease are predominantly associated with serotonin and dopamine. Prog Neuro-Psychopharmacol Biol Psychiatry 34:1104–1114
Hernandez-Baltazar D, Zavala-Flores LM, Villanueva-Olivo A (2017) The 6-hydroxydopamine model and Parkinsonian pathophysiology: Novel findings in an older model. Neurologia 32:533–539
Jeon BS, Jackson-Lewis V, Burke RE (1995) 6-Hydroxydopamine lesion of the rat substantia nigra: time course and morphology of cell death. Neurodegeneration 4:131–137
Lev N, Barhum Y, Ben-Zur T, Melamed E, Steiner I, Offen D (2013) Knocking out DJ-1 attenuates astrocytes neuroprotection against 6-hydroxydopamine toxicity. J Mol Neurosci 50:542–550. https://doi.org/10.1007/s12031-013-9984-9
Ungerstedt U (1968) 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol 5:107–110. https://doi.org/10.1016/0014-2999(68)90164-7
Hamadjida A, Frouni I, Kwan C, Huot P (2019) Classic animal models of Parkinson's disease: A historical perspective. Behav Pharmacol 30:291–310
Tan SH, Karri V, Tay NWR, Chang KH, Ah HY, Ng PQ, Ho HS, Keh HW et al (2019) Emerging pathways to neurodegeneration: dissecting the critical molecular mechanisms in Alzheimer’s disease, Parkinson’s disease. Biomed Pharmacother 111:765–777
Olanow CW, Schapira AHV (2013) Therapeutic prospects for Parkinson disease. Ann Neurol 74:337–347. https://doi.org/10.1002/ana.24011
Garg A, Garg S, Zaneveld LJD, Singla AK (2001) Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phyther Res 15:655–669
Antunes MS, Jesse CR, Ruff JR, de Oliveira Espinosa D, Gomes NS, Altvater EET, Donato F, Giacomeli R et al (2016) Hesperidin reverses cognitive and depressive disturbances induced by olfactory bulbectomy in mice by modulating hippocampal neurotrophins and cytokine levels and acetylcholinesterase activity. Eur J Pharmacol 789:411–420. https://doi.org/10.1016/j.ejphar.2016.07.042
Donato F, de Gomes MG, Goes ATR, Filho CB, del Fabbro L, Antunes MS, Souza LC, Boeira SP et al (2014) Hesperidin exerts antidepressant-like effects in acute and chronic treatments in mice: possible role of l-arginine-NO-cGMP pathway and BDNF levels. Brain Res Bull 104:19–26. https://doi.org/10.1016/j.brainresbull.2014.03.004
Gaur V, Kumar A (2010) Hesperidin pre-treatment attenuates NO-mediated cerebral ischemic reperfusion injury and memory dysfunction. Pharmacol Rep 62:635–648. https://doi.org/10.1016/S1734-1140(10)70321-2
kheradmand E, Hajizadeh Moghaddam A, Zare M (2018) Neuroprotective effect of hesperetin and nano-hesperetin on recognition memory impairment and the elevated oxygen stress in rat model of Alzheimer’s disease. Biomed Pharmacother 97:1096–1101. https://doi.org/10.1016/j.biopha.2017.11.047
Antunes MS, Goes ATR, Boeira SP, Prigol M, Jesse CR (2014) Protective effect of hesperidin in a model of Parkinson’s disease induced by 6-hydroxydopamine in aged mice. Nutrition 30:1415–1422. https://doi.org/10.1016/j.nut.2014.03.024
Carlsson T, Schindler FR, Höllerhage M, Depboylu C, Arias-Carrión O, Schnurrbusch S, Rösler TW, Wozny W et al (2011) Systemic administration of neuregulin-1β1 protects dopaminergic neurons in a mouse model of Parkinson’s disease. J Neurochem 117:1066–1074. https://doi.org/10.1111/j.1471-4159.2011.07284.x
Ungerstedt U (1971) Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand 367:69–93
Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463:3–33
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167. https://doi.org/10.1016/0165-0270(85)90031-7
Clénet F, Bouyon E, Hascoët M, Bourin M (2006) Light/dark cycle manipulation influences mice behaviour in the elevated plus maze. Behav Brain Res 166:140–149. https://doi.org/10.1016/j.bbr.2005.07.018
Yalcin I, Aksu F, Belzung C (2005) Effects of desipramine and tramadol in a chronic mild stress model in mice are altered by yohimbine but not by pindolol. Eur J Pharmacol 514:165–174. https://doi.org/10.1016/j.ejphar.2005.03.029
D’Audiffret AC, Frisbee SJ, Stapleton PA et al (2010) Depressive behavior and vascular dysfunction: a link between clinical depression and vascular disease? J Appl Physiol 108:1041–1051. https://doi.org/10.1152/japplphysiol.01440.2009
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Aguiar AS Jr, Duzzioni M, Remor AP, Tristão FSM, Matheus FC, Raisman-Vozari R, Latini A, Prediger RD (2016) Moderate-intensity physical exercise protects against experimental 6-hydroxydopamine-induced Hemiparkinsonism through Nrf2-antioxidant response element pathway. Neurochem Res 41:64–72
Franklin K, Paxinos G (1997) The mouse brain in stereotaxic coordinates. Academic Press, New York
Oorschot DE (1996) Total number of neurons in the neostriatal, pallidal, subthalamic, and substantia nigral nuclei of the rat basal ganglia: a stereological study using the cavalieri and optical disector methods. J Comp Neurol 366:580–599
Filichia E, Shen H, Zhou X, Qi X, Jin K, Greig N, Hoffer B, Luo Y (2015) Forebrain neuronal specific ablation of p53 gene provides protection in a cortical ischemic stroke model. Neuroscience 295:1–10. https://doi.org/10.1016/j.neuroscience.2015.03.018
Gundersen HJG, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, MØLler A, Nielsen K et al (1988) The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. Apmis 96:857–881. https://doi.org/10.1111/j.1699-0463.1988.tb00954.x
Gundersen HJG (2002) The smooth fractionator. J Microsc 207:191–210. https://doi.org/10.1046/j.1365-2818.2002.01054.x
Schmitz C, Hof PR (2005) Design-based stereology in neuroscience. Neuroscience 130:813–831
Akhmadeeva GN, Magzhanov RV, Tayupova GN, Baitimerov AR, Khidiyatova IM (2018) Depression and anxiety in Parkinson’s disease. Neurosci Behav Physiol 48:636–640. https://doi.org/10.1007/s11055-018-0609-1
Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR (2011) The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord 26:399–406. https://doi.org/10.1002/mds.23462
Willner P (2005) Chronic mild stress (CMS) revisited: Consistency and behavioural- neurobiological concordance in the effects of CMS. Neuropsychobiology 52:90–110
Hirsch EC, Vyas S, Hunot S (2012) Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord 18:S210–S212. https://doi.org/10.1016/s1353-8020(11)70065-7
Wang Q, Liu Y, Zhou J (2015) Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener 4:19
Machado V, Zöller T, Attaai A, Spittau B (2016) Microglia-mediated neuroinflammation and neurotrophic factor-induced protection in the MPTP mouse model of Parkinson’s disease-lessons from transgenic mice. Int J Mol Sci 17:151
Goes ATR, Jesse CR, Antunes MS, Lobo Ladd FV, Lobo Ladd AAB, Luchese C, Paroul N, Boeira SP (2018) Protective role of chrysin on 6-hydroxydopamine-induced neurodegeneration a mouse model of Parkinson’s disease: Involvement of neuroinflammation and neurotrophins. Chem Biol Interact 279:111–120. https://doi.org/10.1016/j.cbi.2017.10.019
Del Fabbro L, Rossito Goes A, Jesse CR et al (2019) Chrysin protects against behavioral, cognitive and neurochemical alterations in a 6-hydroxydopamine model of Parkinson’s disease. Neurosci Lett 706:158–163. https://doi.org/10.1016/j.neulet.2019.05.036
Simon DK, Tanner CM, Brundin P (2020) Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med 36:1–12
Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M (2015) Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phyther Res 29:323–331
Hajialyani M, Farzaei MH, Echeverría J et al (2019) Hesperidin as a neuroprotective agent: a review of animal and clinical evidence. Molecules 24:648
Aron L, Klein R (2011) Repairing the Parkinsonian brain with neurotrophic factors. Trends Neurosci 34:88–100
Sidorova YA, Volcho KP, Salakhutdinov NF (2018) Neuroregeneration in Parkinson’s disease: From proteins to small molecules. Curr Neuropharmacol 17:268–287. https://doi.org/10.2174/1570159x16666180905094123
Li CF, Chen SM, Chen XM, Mu RH, Wang SS, Geng D, Liu Q, Yi LT (2016) ERK-dependent brain-derived neurotrophic factor regulation by hesperidin in mice exposed to chronic mild stress. Brain Res Bull 124:40–47. https://doi.org/10.1016/j.brainresbull.2016.03.016
Hyman C, Juhasz M, Jackson C, Wright P, Ip NY, Lindsay RM (1994) Overlapping and distinct actions of the neurotrophins BDNF, NT-3, and NT- 4/5 on cultured dopaminergic and GABAergic neurons of the ventral mesencephalon. J Neurosci 14:335–347. https://doi.org/10.1523/jneurosci.14-01-00335.1994
Espejo M, Cutillas B, Arenas E, Ambrosio S (2000) Increased survival of dopaminergic neurons in striatal grafts of fetal ventral mesencephalic cells exposed to neurotrophin-3 or glial cell line- derived neurotrophic factor. Cell Transplant 9:45–53. https://doi.org/10.1177/096368970000900107
Hirsch EC (1994) Biochemistry of Parkinson’s disease with special reference to the dopaminergic systems. Mol Neurobiol 9:135–142. https://doi.org/10.1007/BF02816113
Haavik J, Toska K (1998) Tyrosine hydroxylase and Parkinson’s disease. Mol Neurobiol 16:285–309. https://doi.org/10.1007/BF02741387
Kozina EA, Khakimova GR, Khaindrava VG, Kucheryanu VG, Vorobyeva NE, Krasnov AN, Georgieva SG, Kerkerian-le Goff L et al (2014) Tyrosine hydroxylase expression and activity in nigrostriatal dopaminergic neurons of MPTP-treated mice at the presymptomatic and symptomatic stages of parkinsonism. J Neurol Sci 340:198–207. https://doi.org/10.1016/j.jns.2014.03.028
Chaudhuri KR, Schapira AH (2009) Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 8:464–474
Draoui A, El Hiba O, Aimrane A, El Khiat A, Gamrani H (2020) Parkinson's disease: from bench to bedside. Rev Neurol (Paris). https://doi.org/10.1016/j.neurol.2019.11.002
Heinz A, Schmidt LG, Reischies FM (1994) Anhedonia in schizophrenic, depressed, or alcohol-dependent patients–neurobiological correlates. Pharmacopsychiatry 27:7–10
Acknowledgments
The authors are grateful for the financial support by FAPERGS, CNPQ, and CAPES. LCS is recipient by CNPq fellowship (150560/2019-2) and VCB, MRPS, and SMA are recipient by CAPES fellowship (Finance code 001). We would like to thank Professor Cristiano Ricardo Jesse for the study design, interpretation of the data, and for providing their thoughts and his contribution to the development of the experiments.
Funding
This study was financial supported by FAPERGS Research Grants No.16/2551-0000526-5 (PRONUPEQ) and No. 16/2251-0000183-9 (ARD/PPP).
Author information
Authors and Affiliations
Contributions
MSA carried out the experiments and wrote the manuscript. FVLL, AABLL and ALM carried out the experiments. VCB, MRPS and SMA performed behavioral studies and carried out experiments. LCS, participated in the design of the study and experiments, wrote and reviewed the manuscript. MP, CWN and SPB conceived of and participated in the design of the study and reviewed the manuscript. All authors read and approved of the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Specifically, all experiments in the present study were approved by Ethical Committee for Animal Use (CEUA protocol No. 001/2013) of the Federal University of Pampa, Brazil.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Antunes, M.S., Cattelan Souza, L., Ladd, F.V.L. et al. Hesperidin Ameliorates Anxiety-Depressive-Like Behavior in 6-OHDA Model of Parkinson’s Disease by Regulating Striatal Cytokine and Neurotrophic Factors Levels and Dopaminergic Innervation Loss in the Striatum of Mice. Mol Neurobiol 57, 3027–3041 (2020). https://doi.org/10.1007/s12035-020-01940-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01940-3