Abstract
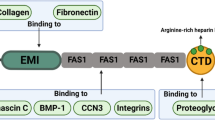
Periostin is a secretory protein with a multi-domain structure, comprising an amino-terminal cysteine-rich EMI domain, four internal FAS 1 domains, and a carboxyl-terminal hydrophilic domain. These adjacent domains bind to extracellular matrix proteins (type I collagen, fibronectin, tenascin-C, and laminin γ2), and BMP-1 that catalyzes crosslinking of type I collagen, and proteoglycans, which play a role in cell adhesion. The binding sites on periostin have been demonstrated to contribute to the mechanical strength of connective tissues, enhancing intermolecular interactions in close proximity and their assembly into extracellular matrix architectures, where periostin plays further essential roles in physiological maintenance and pathological progression. Furthermore, periostin also binds to Notch 1 and CCN3, which have functions in maintenance of stemness, thus opening up a new field of periostin action.



Similar content being viewed by others
References
Bonnet N, Brun J, Rousseau J-C, Duong LT, Ferrari SL (2017) Cathepsin K controls cortical bone formation by degrading periostin. J Bone Miner Res 7:1432–1441
Bozyk PD, Bentley JK, Popova AP, Anyanwu AC, Linn MD, Goldsmith AM, Pryhuber GS, Moore BB, Hershenson MB (2012) Neonatal periostin knockout mice are protected from hyperoxia-induced alveolar simplication. PLoS One 7:e31336. https://doi.org/10.1371/journal.pone.0031336
Canty EG, Kadler KE (2005) Procollagen trafficking, processing and fibrillogenesis. J Cell Sci 118(Pt 7):1341–1353. https://doi.org/10.1242/jcs.01731
Conway SJ, Izuhara K, Kudo Y, Litvin J, Markwald R, Ouyang G, Arron JR, Holweg CT, Kudo A (2014) The role of periostin in tissue remodeling across health and disease. Cell Mol Life Sci 71:1279–1288. https://doi.org/10.1007/s00018-013-1494-y
Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR et al (2011) Lebrikizumab treatment in adults with asthma. N Engl J Med 365:1088–1098
Dai Q, Xie F, Han Y, Ma X, Zhou S, Jiang L, Zou W, Wang J (2017) Inactivation of regulatory-associated protein of mTOR (Raptor)/mammalian target of rapamycin complex 1 (mTORC1) signaling in osteoclasts increases bone mass by inhibiting osteoclast differentiation in mice. J Biol Chem 292:196–204
Djokic J, Fagotto-Kaufmann C, Bartels R, Nelea V, Reinhardt DP (2013) Fiblin-3,-4, and-5 are highly susceptible to proteolysis, interact with cells and heparin, and form multimers. J Biol Chem 288:22821–22835
Elliott CG, Hamilton DW (2011) Deconstructing fibrosis research: do pro-fibrotic signals point the way for chronic dermal wound regeneration? J Cell Commun Signal 5:301–315. https://doi.org/10.1007/s12079-011-0131-5
Fukushima N, Kikuchi Y, Nishiyama T, Kudo A, Fukayama M (2008) Periostin deposition in the stroma of invasive and intraductal neoplasms of the pancreas. Mod Pathol 21:1044–1053. https://doi.org/10.1038/modpathol.2008.77
Garnero P (2012) The contribution of collagen crosslinks to bone strength. Bonekey Rep 1:182. https://doi.org/10.1038/bonekey.2012.182
Gupta R, Hong D, Iborra F, Sarno S, Enver T (2007) NOV (CCN3) functions as regulator of human hematopietic stem of progenitor cells. Science 316:590–593
Hakuno D, Kimura N, Yoshioka M, Mukai M, Kimura T, Okada Y, Yozu R, Shukunami C, Hiraki Y, Kudo A, Ogawa S, Fukuda K (2010) Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J Clin Invest 120:2292–2306. https://doi.org/10.1172/JCI40973
Hashimoto K, Noshiro M, Ohno S, Kawamoto T, Satakeda H, Akagawa Y, Nakashima K, Okimura A, Ishida H, Okamoto T, Pan H, Shen M, Yan W, Kato Y (1997) Characterization of a cartilage-derived 66-kDa protein (RGD-CAP/beta ig-h3) that binds to collagen. Biochim Biophys Acta 1355:303–314
Hoersch S, Andrade-Navarro MA (2010) Periostin shows increased evolutionary plasticity in its alternatively spliced region. BMC Evol Biol 10:30. https://doi.org/10.1186/1471-2148-10-30
Horiguchi M, Inoue T, Ohbayashi T, Hirai M, Noda K, Marmorstein LY, Yabe D, Takagi K, Akama TO, Kita T, Kimura T, Nakamura T (2009) Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc Natl Acad Sci U S A 45:19029–19034
Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A (1999) Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 14:1239–1249. https://doi.org/10.1359/jbmr.1999.14.7.1239
Hwang EY, Jeong MS, Park EK, Kim JH, Jang SB (2014) Structural characterization and interaction of periostin and bone morphogenetic protein for regulation of collagen cross-linking. Biochem Biophys Res Commun 449:425–431. https://doi.org/10.1016/j.bbrc.2014.05.055
Ishihara J, Umemoto T, Yamato M, Shiratsuchi Y, Takaki S, Petrich BG, Nakauchi H, Eto K, Kitamura T, Okano T (2014) Nov/CCN3 regulates long-term repopulating activity of murine hematopoietic stem cells via integrin avb3. Int J Hematol 99:393–406
Ishikawa K, Yoshida S, Nakao S, Nakama T, Kita T, Asato R, Sassa Y, Arita R, Miyazaki M, Enaida H, Oshima Y, Murakami N, Niiro H, Ono J, Matsuda A, Goto Y, Akashi K, Izuhara K, Kudo A, Kono T, Hafezi-Moghadam A, Ishibashi T (2014) Periostin promotes the generation of fibrous membranes in proliferative vitreoretinopathy. FASEB J 28:131–142. https://doi.org/10.1096/fj.13-229740
Ito N, Ruegg UT, Kudo A, Miyagoe-Suzuki Y, Takeda S (2013) Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat Med 19:101–106
Kadler KE, Hill A, Canty-Laird EG (2008) Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol 20:495–501. https://doi.org/10.1016/j.ceb.2008.06.008
Kashima TG, Nishiyama T, Shimazu K, Shimazaki M, Kii I, Grigoriadis AE, Fukayama M, Kudo A (2009) Periostin, a novel marker of intramembranous ossification, is expressed in fibrous dysplasia and in c-Fos-overexpressing bone lesions. Hum Pathol 40:226–237. https://doi.org/10.1016/j.humpath.2008.07.008
Khurana S, Schouteden S, Manesia JK, Sanamaria-Martinez A, Huelsken J, Lacy-Hulbert A, Verfaillie CM (2016) Outside-in integrin signaling regulates haematopoietic stem cell function via Periostin-Itgav axis. Nat Commun 7:13500
Kii I, Amizuka N, Minqi L, Kitajima S, Saga Y, Kudo A (2006) Periostin is an extracellular matrix protein required for eruption of incisors in mice. Biochem Biophys Res Commun 342:766–772. https://doi.org/10.1016/j.bbrc.2006.02.016
Kii I, Nishiyama T, Li M, Matsumoto K, Saito M, Amizuka N, Kudo A (2010) Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem 285:2028–2039. https://doi.org/10.1074/jbc.M109.051961
Kii I, Nishiyama T, Kudo A (2016) Periostin promotes secretion of fibronectin from the endoplasmic reticulum. Biochem Biophys Res Commun 470:888–893. https://doi.org/10.1016/j.bbrc.2016.01.139
Kikuchi Y, Kashima TG, Nishiyama T, Shimazu K, Morishita Y, Shimazaki M, Kii I, Horie H, Nagai H, Kudo A, Fukayama M (2008) Periostin is expressed in pericryptal fibroblasts and cancer-associated fibroblasts in the colon. J Histochem Cytochem 56:753–764. https://doi.org/10.1369/jhc.2008.951061
Kikuchi Y, Kunita A, Iwata C, Komura D, Nishiyama T, Shimazu K, Takeshita K, Shibahara J, Kii I, Morishita Y, Yashiro M, Hirakawa K, Miyazono K, Kudo A, Fukayama M, Kashima TG (2014) The niche component periostin is produced by cancer-associated fibroblasts, supporting growth of gastric cancer through ERK activation. Am J Pathol 184:859–870. https://doi.org/10.1016/j.ajpath.2013.11.012
Kim BY, Olzmann JA, Choi SI, Ahn SY, Kim TI, Cho HS, Suh H, Kim EK (2009) Corneal dystrophy-associated R124H mutation disrupts TGFBI interaction with Periostin and causes mislocalization to the lysosome. J Biol Chem 284:19580–19591. https://doi.org/10.1074/jbc.M109.013607
Kim H-G, Hwang S-Y, Aaronson SA, Mandinova A, Lee SW (2011) DDR1 receptor tyrosin kinase promotes prosurvival pathway through notch 1 activation. J Biol Chem 286:17672–17681
Kondoh H, Nishiyama T, Kikuchi Y, Fukayama M, Saito M, Kii I, Kudo A (2016) Periostin deficiency causes severe and lethal lung injury in mice with bleomycin administration. J Histochem Cytochem 64:441–453. https://doi.org/10.1369/0022155416652611
Kraft M (2011) Asthma phenotypes and interleukin-13--moving closer to personalized medicine. N Engl J Med 365:1141–1144
Kudo A (2011) Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci 68:3201–3207. https://doi.org/10.1007/s00018-011-0784-5
Liu AY, Zheng H, Ouyang G (2014) Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments. Matrix Biol 37:150–156. https://doi.org/10.1016/j.matbio.2014.04.007
Maruhashi T, Kii I, Saito M, Kudo A (2010) Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J Biol Chem 285:13294–13303. https://doi.org/10.1074/jbc.M109.088864
Merie B, Bouet G, Rousseau J-C, Betholon C, Garnero P (2014) Periostin and transforming growth factor β-induced protein (TGFβIp) are both expressed by osteoblasts and osteoclasts. Cell Biol Int 38:398–404
Midwood KS, Chiquet M, Tucker RP, Orend G (2016) Tenascin-C at a glance. J Cell Sci 129:4321–4327. https://doi.org/10.1242/jcs.190546
Naik PK, Bozyk PD, Bentley JK, Popova AP, Birch CM, Wilke CA, Fry CD, White ES, Sisson TH, Tayob N, Carnemolla B, Orecchia P, Flaherty KR, Hershenson MB, Murray S, Martinez FJ, Moore BB, Investigators C (2012) Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 303:L1046–L1056. https://doi.org/10.1152/ajplung.00139.2012
Nakama T, Yoshida S, Ishikawa K, Kobayashi Y, Zhou Y, Nakao S, Sassa Y, Oshima Y, Takao K, Shimahara A, Yoshikawa K, Hamasaki T, Ohgi T, Hayashi H, Matsuda A, Kudo A, Nozaki M, Ogura Y, Kuroda M, Ishibashi T (2015) Gene Ther 22:127–137
Nakama T, Yoshida S, Ishikawa K, Kubo Y, Kobayashi Y, Zhou Y, Nakao S, Hisatomi T, Ikeda Y, Takao K, Yoshikawa K, Matsuda A, Ono J, Ohta S, Izuhara K, Kudo A, Sonoda K, Ishibashi T (2017) Mol Ther-Nucleic Acids 6:279–289
Nam BY, Park JT, Kwon YE, Lee JP, Jung JH, Kim Y, Kim S, Park J, Um JE, Wu M, Han SH, Yoo T-H, Kang S-W (2017) Mol Ther Nucleic Acids. doi: https://doi.org/10.1016/j.omtn.2017.05.001
Nishiyama T, Kii I, Kashima TG, Kikuchi Y, Ohazama A, Shimazaki M, Fukayama M, Kudo A (2011) Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS One 6:e18410. https://doi.org/10.1371/journal.pone.0018410
Nitsche U, Stangel D, Pan Z, Schlitter AM, Esposito I, Regel I, Raulefs S, Friess H, Kleeff J, Erkan M (2016) Periostin and tumor-stroma interactions in non-small cell lung cancer. Oncol Lett 12:3804–3810. https://doi.org/10.3892/ol.2016.5132
Noack S, Seiffart V, Willbold E, Laggies S, Winkel A, Shahab-Osterloh S, Florkemeier T, Hertwig F, Steinhoff C, Nuber UA, Gross G, Hoffmann A (2014) Periostin secreted by mesenchymal stem cells supports tendon formation in an ectopic mouse model. Stem Cells Dev 23:1844–1857
Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, Hoffman S, Wen X, Sugi Y, Kern CB, Mjaatvedt CH, Turner DK, Oka T, Conway SJ, Molkentin JD, Forgacs G, Markwald RR (2007) Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem 101:695–711. https://doi.org/10.1002/jcb.21224
Norris RA, Moreno-Rodriguez R, Hoffman S, Markwald RR (2009a) The many facets of the matricelluar protein periostin during cardiac development, remodeling, and pathophysiology. J Cell Commun Signal 3:275–286. https://doi.org/10.1007/s12079-009-0063-5
Norris RA, Potts JD, Yost MJ, Junor L, Brooks T, Tan H, Hoffman S, Hart MM, Kern MJ, Damon B, Markwald RR, Goodwin RL (2009b) Periostin promotes a fibroblastic lineage pathway in atrioventricular valve progenitor cells. Dev Dyn 238:1052–1063. https://doi.org/10.1002/dvdy.21933
Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD (2007) Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res 101: 313–321. doi:https://doi.org/10.1161/CIRCRESAHA.107.149047
Okamoto M, Hoshino T, Kitasato Y, Sakazaki Y, Kawayama T, Fujimoto K, Ohshima K, Shiraishi H, Uchida M, Ono J, Ohta S, Kato S, Izuhara K, Aizawa H (2011) Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur Respir J 37:1119–1127. https://doi.org/10.1183/09031936.00059810
Ontsuka K, Kotobuki Y, Shiraishi H, Serada S, Ohta S, Tanemura A, Yang L, Fujimoto M, Arima K, Suzuki S, Murota H, Toda S, Kudo A, Conway SJ, Narisawa Y, Katayama I, Izuhara K, Naka T (2012) Periostin, a matricellular protein, accelerates cutaneous wound repair by activating dermal fibroblasts. Exp Dermatol 21:331–336. https://doi.org/10.1111/j.1600-0625.2012.01454.x
Oskarsson T, Massague J (2012) Extracellular matrix players in metastatic niches. EMBO J 31:254–256. https://doi.org/10.1038/emboj.2011.469
Polizzotti BD, Arab S, Kuhn B (2012) Intrapericardial delivery of gelform enables the targeted delivery of periostin peptide after myocardial infarction by inducing fibrin clot formation. PLoS One 7:e36788
Prakoura N, Chatziantoniou C (2017) Periostin and discoidin domain receptor 1: new biomarkers or targets for therapy of renal disease. Front Med 4:52
Qin X, Yan M, Zhang J, Wang X, Shen Z, Lv Z, Li Z, Wei W, Chen W (2016) TGFbeta3-mediated induction of Periostin facilitates head and neck cancer growth and is associated with metastasis. Sci Rep 6:20587. https://doi.org/10.1038/srep20587
Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A, Rogers R, Chen Z, Maeda M, Kruzynska-Frejtag A, Feng JQ, Conway SJ (2005) Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol 25:11131–11144. https://doi.org/10.1128/MCB.25.24.11131-11144.2005
Rosselli-Murai LK, Almeida LO, Zagni C, Galindo-Moreno P, Padial-Molina M, Volk SL, Murai MJ, Rios HF, Squarize CH, Castilho RM (2013) Periostin responds to mechanical stress and tension by activating the MTOR signaling pathway. PLoS One 8:e83580
Ruan K, Bao S, Ouyang G (2009) The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci 66:2219–2230. https://doi.org/10.1007/s00018-009-0013-7
Schwanekamp JA, Lorts A, Sargent MA, York AJ, Grimes KM, Fischesser DM, Gokey JJ, Whitsett JA, Conway SJ, Molkentin JD (2017) TGFB1 functiones similar to periostin but is uniquely dispensable during cardiac injury. PLoS One. https://doi.org/10.1371/journal.pone.0181945
Sens C, Huck K, Pettera S, Uebel S, Wabnitz G, Moser M, Nakchbandi IA (2016) Fibronectin containing extradomain A or B enhance osteoblast differentiation via distinct integrins. J Biol Chem 292:7745–7760
Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, Saito M, Fukuda K, Nishiyama T, Kitajima S, Saga Y, Fukayama M, Sata M, Kudo A (2008) Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med 205:295–303. https://doi.org/10.1084/jem.20071297
Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L, Firulli AB, Molkentin JD, Markwald R, Conway SJ (2008) Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res 102:752–760. https://doi.org/10.1161/CIRCRESAHA.107.159517
Sugiura T, Takamatsu H, Kudo A, Amann E (1995) Expression and characterization of murine osteoblast-specific factor 2 (OSF-2) in a baculovirus expression system. Protein Expr Purif 6:305–311. https://doi.org/10.1006/prep.1995.1040
Sung PL, Jan YH, Lin SC, Huang CC, Lin H, Wen KC, Chao KC, Lai CR, Wang PH, Chuang CM, HH W, Twu NF, Yen MS, Hsiao M, Huang CY (2016) Periostin in tumor microenvironment is associated with poor prognosis and platinum resistance in epithelial ovarian carcinoma. Oncotarget 7:4036–4047. 10.18632/oncotarget.6700
Suzuki H, Amizuka N, Kii I, Kawano Y, Nozawa-Inoue K, Suzuki A, Yoshie H, Kudo A, Maeda T (2004) Immunohistochemical localization of periostin in tooth and its surrounding tissues in mouse mandibles during development. Anat Rec A Discov Mol Cell Evol Biol 281:1264–1275. https://doi.org/10.1002/ar.a.20080
Takai S, Yoshino M, Takao K, Yoshikawa K, Jin D (2017) Periostin antisense oligonucleotide prevents adhesion formation after surgery in mice. J Pharmacol Sci 133:65–69
Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K (2006) Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol 118:98–104. https://doi.org/10.1016/j.jaci.2006.02.046
Takayama I, Kii I, Kudo A (2009) Expression, purification and characterization of soluble recombinant periostin protein produced by Escherichia coli. J Biochem 146:713–723. https://doi.org/10.1093/jb/mvp117
Takayama I, Tanabe H, Nishiyama T, Ito H, Amizuka N, Li M, Katsube KI, Kii I, Kudo A (2017) Periostin is required for matricellular localization of CCN3 in periodontal ligament of mice. J Cell Commun Signal 11:5–13. https://doi.org/10.1007/s12079-016-0371-5
Tanabe H, Takayama I, Nishiyama T, Shimazaki M, Kii I, Li M, Amizuka N, Katsube K, Kudo A (2010) Periostin associates with Notch 1 precursor to maintain Notch 1 expression under a stress condition in mouse cells. PLoS One 5:e12234
Tanaka S, Maekawa A, Matsubara L, Imanishi A, Yano M, Roeder RG, Hasegawa N, Asano S, Ito M (2016) Periostin supports hematopoietic progenitor cells and niche-dependent myeloblastoma cells in vitro. Biochem Biophys Res Commun 478:1706–1712
Tang Y, Liu L, Wang P, Chen D, Wu Z, Tang C (2017) Periostin promotes migration and osteogenic differentiation of human periodontal ligament mesenchymal stem cells via the Jun amino-terminal kinases (JNK) pathway under inflammatory conditions. Cell Prolif 2017:e12369
Tian Y, Choi CH, Li QK, Rahmatpanah FB, Chen X, Kim SR, Veltri R, Chia D, Zhang Z, Mercola D, Zhang H (2015) Overexpression of periostin in stroma positively associated with aggressive prostate cancer. PLoS One 10:e0121502. https://doi.org/10.1371/journal.pone.0121502
Tomaru A, Kobayashi T, Hinneh JA, Tonto PB, D’Alessandro-Gabazza CN, Fujimoto H, Fujiwara K, Takahashi Y, Ohnishi M, Yasuma T, Nishihama K, Yoshino M, Takao K, Toda M, Totoki T, Takei Y, Yoshikawa K, Taguchi O, Gabazza EC (2017) Oligonucleotides targeting periostin ameliorates pulmonary fibrosis. Gene Ther. https://doi.org/10.1038/gt.2017.80
Trackman PC (2016) Enzymatic and non-enzymatic functions of the lysyl oxidase family in bone. Matrix Biol 52-54:7–18. https://doi.org/10.1016/j.matbio.2016.01.001
Uchida M, Shiraishi H, Ohta S, Arima K, Taniguchi K, Suzuki S, Okamoto M, Ahlfeld SK, Ohshima K, Kato S, Toda S, Sagara H, Aizawa H, Hoshino T, Conway SJ, Hayashi S, Izuhara K (2012) Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol Biol 46:677–686. https://doi.org/10.1165/rcmb.2011-0115OC
Underwood TJ, Hayden AL, Derouet M, Garcia E, Noble F, White MJ, Thirdborough S, Mead A, Clemons N, Mellone M, Uzoho C, Primrose JN, Blaydes JP, Thomas GJ (2015) Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma. J Pathol 235:466–477. https://doi.org/10.1002/path.4467
Vadon-Le Goff S, Hulmes DJ, Moali C (2015) BMP-1/tolloid-like proteinases synchronize matrix assembly with growth factor activation to promote morphogenesis and tissue remodeling. Matrix Biol 44-46:14–23. https://doi.org/10.1016/j.matbio.2015.02.006
Vico L, Rietbergen BV, Vilayphiou N, Linossier M-T, Locrelle H, Normand M, Zouch M, Gerbaix M, Bonnet N, Novikov V, Thomas T, Vassilieva G (2017) Cortical and trabecular bone microstructure did not recover at weight-bearing skeletal sites and progressively deteriorated at non-weight-bearing sites during the year following International Space Station missions. J Bone Miner Res. https://doi.org/10.1002/jbmr.3188
Wang Z, Ouyang G (2012) Periostin: a bridge between cancer stem cells and their metastatic niche. Cell Stem Cell 10:111–112. https://doi.org/10.1016/j.stem.2012.01.002
Zhang T, Ma G, Zhang Y, Huo H, Zhao Y (2017) miR-599 inhibits proliferation and invasion of glioma by targeting periostin. Biotechnol Lett. https://doi.org/10.1007/s10529-017-2365-7
Zhou HM, Wang J, Elliott C, Wen W, Hamilton DW, Conway SJ (2010) Spatiotemporal expression of periostin during skin development and incisional wound healing: lessons for human fibrotic scar formation. J Cell Commun Signal 4:99–107. https://doi.org/10.1007/s12079-010-0090-2
Zhu R, Zheng Y, Dirks NL, Vadhavkar S, Jin JY, Peng K, Holweg CTJ, Olsson J, Matthews JG, Putnam WS (2017) Model-based clinical pharmacological profiling and exposure-response relationships of the efficacy and biomarker of lebrikizumab in patients with moderate-to-serve asthma. Pulm Pharmacol Ther. https://doi.org/10.1016/j.pupt.2017.08.010
Acknowledgements
We thank K. Yoshikawa in Aqua Therapeutics Co. for providing information. This work was supported by the Project for Cancer Research and Therapeutic Evolution (P-CREATE) (IK) from the Japan Agency for Medical Research and Development (AMED).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kudo, A., Kii, I. Periostin function in communication with extracellular matrices. J. Cell Commun. Signal. 12, 301–308 (2018). https://doi.org/10.1007/s12079-017-0422-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-017-0422-6