Abstract
Objective
The aim of this study was to measure the apparent diffusion coefficient (ADC) value at the region with the highest FDG uptake using sequential 18F-FDG PET and MRI, and to correlate it with the histological grade of invasive ductal carcinoma (IDC) of the breast.
Methods
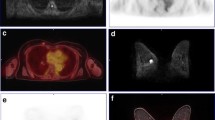
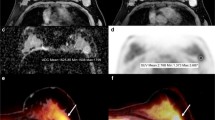
A retrospective study was conducted on 75 untreated patients with IDC. First, a PET/CT scan and subsequent breast MRI were done and the SUVmax of the each breast tumor was recorded. Then, a PET image and ADC map were co-registered. On the axial slice containing the pixel with SUVmax, we drew multiple circular ROIs within the tumor and measured the mean ADC value of each ROI. The average (ADC-mean) and minimum (ADC-min) of the mean ADC values for all ROIs within the tumor were calculated, respectively. Then, a circular ROI was placed at the corresponding location to the pixel with the highest SUV and the mean ADC value of the ROI was denoted as ADC-PET. We compared the averages of the ADC parameters and assessed the correlations among SUVmax and ADC parameters. ROC curve and logistic regression analyses were performed to assess the utility of ADC and SUVmax for detecting histological grade 3.
Results
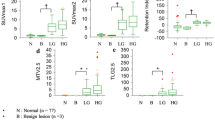
ADC-min was significantly lower than the ADC-mean or ADC-PET. All of the ADC parameters showed a negative correlation with SUVmax. The area under the ROC curve for identifying histological grade 3 using ADC-PET, ADC-min, ADC-mean and SUVmax was 0.684, 0.660, 0.633 and 0.639, respectively. By multivariate analysis, ADC-PET was a significant, independent predictor of histological grade 3 (p = 0.004).
Conclusions
We estimated the ADC value at the breast tumor region with the highest FDG uptake using sequential 18F-FDG PET and MRI. This new ADC parameter distinguished high-grade IDC, supporting the feasibility of the combined PET-MRI system in patients with breast cancer.





Similar content being viewed by others
References
Choi SY, Chang YW, Park HJ, Kim HJ, Hong SS, Seo DY. Correlation of diffusion-weighted imaging apparent diffusion coefficient with prognostic factors of breast cancer. Br J Radiol. 2012;85:e474–9.
Hirano M, Satake H, Ishigaki S, Ikeda M, Kawai H, Naganawa S. Diffusion-weighted imaging of breast masses: comparison of diagnostic performance using various apparent diffusion coefficient parameters. Am J Roentgenol. 2012;198:717–22.
Razek AA, Gaballa G, Denewer A, Nada N. Invasive ductal carcinoma: correlation of apparent diffusion coefficient value with pathological prognostic factors. NMR Biomed. 2010;23:619–23.
Oshida M, Uno K, Suzuki M, Nagashima T, Hashimoto H, Yagata H, et al. Predicting the prognoses of breast carcinoma patients with positron emission tomography using 2-deoxy-2-fluoro[18F]-d-glucose. Cancer. 1998;82:2227–34.
Inoue T, Yutani K, Taguchi T, Tamaki Y, Shiba E, Noguchi S. Preoperative evaluation of prognosis in breast cancer patients by [(18)F]2-deoxy-2-fluoro-d-glucose-positron emission tomography. J Cancer Res Clin Oncol. 2004;130:273–8.
Nakajo M, Kajiya Y, Kaneko T, Kaneko Y, Takasaki T, Tani A, et al. FDG PET/CT and diffusion-weighted imaging for breast cancer: prognostic value of maximum standardized uptake values and apparent diffusion coefficient values of the primary lesion. Eur J Nucl Med Mol Imaging. 2010;37:2011–20.
Drzezga A, Souvatzoglou M, Eiber M, Beer AJ, Fürst S, Martinez-Möller A, et al. First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. J Nucl Med. 2012;53:845–55.
Zaidi H, Ojha N, Morich M, Hu Z, Maniawski P, Ratib O, et al. Design and performance evaluation of a whole-body Ingenuity TF PET-MRI system. Phys Med Biol. 2011;56:3091–106.
Delso G, Ziegler S. PET/MRI system design. Eur J Nucl Med Mol Imaging. 2009;36(Suppl):S86–92.
Buchbender C, Heusner TA, Lauenstein TC, Bockisch A, Antoch G. Oncologic PET/MRI, part 1: tumors of the brain, head and neck, chest, abdomen, and pelvis. J Nucl Med. 2012;53:928–38.
Park H, Wood D, Hussain H, Meyer CR, Shah RB, Johnson TD, et al. Introducing parametric fusion PET/MRI of primary prostate cancer. J Nucl Med. 2012;53:546–51.
Hoppenrath M. Understanding multimodal fusion imaging. Appl Radiol. 2004;33:40–7.
Dmitriev ID, Loo CE, Vogel WV, Pengel KE, Gilhuijs KG. Fully automated deformable registration of breast DCE-MRI and PET/CT. Phys Med Biol. 2013;21:1221–33.
Unlu MZ, Krol A, Magri A, Mandel JA, Lee W, Baum KG, et al. Computerized method for nonrigid MR-to-PET breast-image registration. Comput Biol Med. 2010;40:37–53.
Kiefer A, Kuwert T, Hahn D, Hornegger J, Uder M, Ritt P. Anatomical accuracy of abdominal lesion localization. Retrospective automatic rigid image registration between FDG-PET and MRI. Nuklearmedizin. 2011;50:147–54.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10.
Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–43.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
Avril N, Menzel M, Dose J, Schelling M, Weber W, Jänicke F, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med. 2001;42:9–16.
Gil-Rendo A, Martinez-Regueira F, Zornoza G, Garcia-Velloso MJ, Beorlegui C, Rodriguez-Spiteri N. Association between [18F]fluorodeoxyglucose uptake and prognostic parameters in breast cancer. Brit J surg. 2009;96:166–70.
Sanli Y, Kuyumcu S, Ozkan ZG, Işik G, Karanlik H, Guzelbey B, et al. Increased FDG uptake in breast cancer is associated with prognostic factors. Ann Nucl Med. 2012;26:345–50.
Adejolu M, Huo L, Rohren E, Santiago L, Yang WT. False-positive lesions mimicking breast cancer on FDG PET and PET/CT. Am J Roentgenol. 2012;198:W304–14.
Avril N, Rose CA, Schelling M, Dose J, Kuhn W, Bense S, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol. 2000;18:3495–502.
Tsushima Y, Takahashi-Taketomi A, Endo K. Magnetic resonance (MR) differential diagnosis of breast tumors using apparent diffusion coefficient (ADC) on 1.5-T. J Magn Reson Imaging. 2009;30:249–55.
Humphries PD, Sebire NJ, Siegel MJ, Olsen OE. Tumors in pediatric patients at diffusion-weighted MR imaging: apparent diffusion coefficient and tumor cellularity. Radiology. 2007;245:848–54.
Kul S, Cansu A, Alhan E, Dinc H, Gunes G, Reis A. Contribution of diffusion-weighted imaging to dynamic contrast-enhanced MRI in the characterization of breast tumors. Am J Roentgenol. 2011;196:210–7.
Satake H, Nishio A, Ikeda M, Ishigaki S, Shimamoto K, Hirano M, et al. Predictive value for malignancy of suspicious breast masses of BI-RADS categories 4 and 5 using ultrasound elastography and MR diffusion-weighted imaging. Am J Roentgenol. 2011;196:202–9.
Rubesova E, Grell AS, De Maertelaer V, Metens T, Chao SL, Lemort M. Quantitative diffusion imaging in breast cancer: a clinical prospective study. J Magn Reson Imaging. 2006;24:319–24.
Yabuuchi H, Matsuo Y, Okafuji T, Kamitani T, Soeda H, Setoguchi T, et al. Enhanced mass on contrast-enhanced breast MR imaging: lesion characterization using combination of dynamic contrast-enhanced and diffusion-weighted MR images. J Magn Reson Imaging. 2008;28:1157–65.
Tchou J, Sonnad SS, Bergey MR, Basu S, Tomaszewski J, Alavi A, et al. Degree of tumor FDG uptake correlates with proliferation index in triple negative breast cancer. Mol Imaging Biol. 2010;12:657–62.
Shimoda W, Hayashi M, Murakami K, Oyama T, Sunagawa M. The relationship between FDG uptake in PET scans and biological behavior in breast cancer. Breast cancer. 2007;14:260–8.
Acknowledgements
This work was supported by Establishment of Center for PET Application Technology Development, Korea Institute of Radiological and Medical Sciences (KIRAMS), and by Grants from the Ministry of Education, Science and Technology (50441-2012). The authors thank Mr. In Ok Ko and Mr. Young Jun Kim for their excellent technical and generous support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Byun, B.H., Noh, W.C., Lim, I. et al. A new method for apparent diffusion coefficient measurement using sequential 18F-FDG PET and MRI: correlation with histological grade of invasive ductal carcinoma of the breast. Ann Nucl Med 27, 720–728 (2013). https://doi.org/10.1007/s12149-013-0737-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-013-0737-1