Abstract
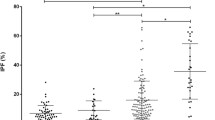
The diagnosis of primary immune thrombocytopenia (ITP) is based on differential diagnosis. Although the measurement of percentages of reticulated platelets (RP%) by flow cytometry is useful as a supportive diagnostic test, this method is nonetheless a time-consuming, laboratory-based assay. To identify alternative assays that are useful in daily practice, we compared three methods in parallel, IPF% measured by XE-2100 [IPF% (XE), Sysmex Corp.], IPF% measured by new XN-1000 [IPF% (XN)], and RP%. We examined 47 patients with primary ITP, 28 patients with aplastic thrombocytopenia (18 aplastic anemia and 10 chemotherapy-induced thrombocytopenia) and 80 healthy controls. In a selected experiment, we examined 16 patients with paroxysmal nocturnal hemoglobinuria (PNH) to examine the effect of hemolysis. As compared with IPF% (XE), IPF% (XN) showed better within-run reproducibility. The sensitivity and specificity for the diagnosis of ITP were 83.0 and 75.0 % for IPF% (XE), 85.1 and 89.3 % for IPF% (XN), and 93.6 and 89.3 % for RP%, respectively. Examination of PNH patients revealed that hemolysis and/or red blood cell fragments interfered with IPF% (XE) values, but not with IFP % (XN) values. Our results suggest that IPF% measured by XN-1000 may be of comparable value with RP% as a supportive diagnostic test for ITP.





Similar content being viewed by others
References
Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995–1008.
McMillan R. The pathogenesis of chronic immune thrombocytopenic purpura. Semin Hematol. 2007;44:S3–11.
Kashiwagi H, Tomiyama Y. Pathophysiology and management of primary immune thrombocytopenia. Int J Hematol. 2013;98:24–33.
Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–93.
Brighton TA, Evans S, Castaldi PA, Chesterman CN, Chong BH. Prospective evaluation of the clinical usefulness of an antigen specific assay (MAIPA) in idiopathic thrombocytopenic purpura and other immune thrombocytopenias. Blood. 1996;88:194–201.
McMillan R, Wang L, Tani P. Prospective evaluation of the immunobead assay for the diagnosis of adult chronic immune thrombocytopenic purpura (ITP). J Thromb Haemost. 2003;1:485–91.
Tomiyama Y, Kosugi S. Autoantigenic epitopes on platelet glycoproteins. Int J Hematol. 2005;81:100–5.
Kosugi S, Kurata Y, Tomiyama Y, Tahara T, Kato T, Tadokoro S, et al. Circulating thrombopoietin level in chronic immune thrombocytopenic purpura. Br J Haematol. 1996;93:704–6.
Emmons RV, Reid DM, Cohen RL, Meng G, Young NS, Dunbar CE, et al. Human thrombopoietin levels are high when thrombocytopenia is due to megakaryocyte deficiency and low when due to increased platelet destruction. Blood. 1996;87:4068–71.
Kurata Y, Hayashi S, Kiyoi T, Kosugi S, Kashiwagi H, Honda S, et al. Diagnostic value of tests for reticulated platelets, plasma glycocalicin, and thrombopoietin levels for discriminating between hyperdestructive and hypoplastic thrombocytopenia. Am J Clin Pathol. 2001;115:656–64.
Kienast J, Schmitz G. Flow cytometric analysis of thiazole orange uptake by platelets: a diagnostic aid in the evaluation of thrombocytopenic disorders. Blood. 1990;75:116–21.
Richards EM, Baglin TP. Quantitation of reticulated platelets: methodology and clinical application. Br J Haematol. 1995;91:445–51.
Kuwana M, Kurata Y, Fujimura K, Fujisawa K, Wada H, Nagasawa T, et al. Preliminary laboratory based diagnostic criteria for immune thrombocytopenic purpura: evaluation by multi-center prospective study. J Thromb Haemost. 2006;4:1936–43.
Barsam SJ, Psaila B, Forestier M, Page LK, Sloane PA, Geyer JT, et al. Platelet production and platelet destruction: assessing mechanisms of treatment effect in immune thrombocytopenia. Blood. 2011;117:5723–32.
Hayashi S, Nishiyama M, Suehisa E, Kashiwagi H, Kurata Y, Tomiyama Y. Comparison between two methods for the measurement reticulated platelet and their clinical significance—flow cytometry (FCM) method and IPF method using automated hematology analyzer (XE-2000). Rinsho Byori. 2009;57:1039–44.
International agranulocytosis and aplastic anemia study. Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood. 1987;70:1718–21.
van der Linden N, Klinkenberg LJ, Meex SJ, Beckers EA, de Wit NC, Prinzen L. Immature platelet fraction measured on the Sysmex XN hemocytometer predicts thrombopoietic recovery after autologous stem cell transplantation. Eur J Haematol. 2014;93:150–6.
Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317:1767–70.
Zhang L, Orban M, Lorenz M, Barocke V, Braun D, Urtz N, et al. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. J Exp Med. 2012;209:2165–81.
Kienast J, Schmitz G. Flow cytometric analysis of thiazole orange uptake by platelets: a diagnostic aid in the evaluation of thrombocytopenic disorders. Blood. 1990;75:116–21.
Tanaka Y, Tanaka Y, Gondo K, Maruki Y, Kondo T, Asai S, et al. Performance evaluation of platelet counting by novel fluorescent dye staining in the XN-series automated hematology analyzers. J Clin Lab Anal. 2014;28:341–8.
Acknowledgments
This work was supported by Grant-in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan and the Ministry of Health, Labor and Welfare in Japan.
Conflict of interest
Automated hematology analyzers, XE-2100 and XN-1000, were supplied by Sysmex Corp. During this study the authors have no other COI to declare.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sakuragi, M., Hayashi, S., Maruyama, M. et al. Clinical significance of IPF% or RP% measurement in distinguishing primary immune thrombocytopenia from aplastic thrombocytopenic disorders. Int J Hematol 101, 369–375 (2015). https://doi.org/10.1007/s12185-015-1741-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-015-1741-0