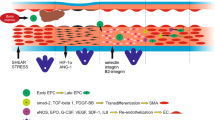
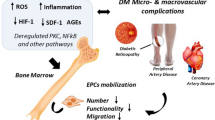
Abstract
Cardiovascular complications have been major concerns in the treatment of diabetes, and up to 80% of all deaths in diabetic patients are linked to cardiovascular problems. Impaired angiogenesis is one of the most serious symptoms associated with diabetes, resulting in delayed wound healing and lower limb amputation. Endothelial progenitor cells (EPCs), a subpopulation of adult stem cells, are recruited from bone marrow to the injured vessel to promote endothelial regeneration and neovascularization, playing an important role in angiogenesis. Interestingly, several clinical studies have showed that the number of recruited EPCs is reduced and their function is decreased under diabetic conditions, implying that diabetic EPC dysfunction may contribute to defective angiogenesis and resultant cardiovascular complications in diabetes. To recover the functional abilities of diabetic EPCs and to address possible application of EPC cell therapy to diabetic patients, some studies provided explanations for diabetic EPC dysfunction including increased oxidative stress, involvement of the inflammatory response, alteration in the nitric oxide pathway and reduced signals for EPC recruitment. This review discusses clinical evidence of impairment of EPC functions under diabetic conditions and the suggested mechanisms for diabetic EPC dysfunction.
Similar content being viewed by others
References
Alam, M. M., Mohammad, A. A., Shuaib, U., Wang, C., Ghani, U., Schwindt, B., Todd, K. G., and Shuaib, A., Homocysteine reduces endothelial progenitor cells in stroke patients through apoptosis. J. Cereb. Blood Flow Metab., 29, 157–165 (2009).
Asahara, T., Murohara, T., Sullivan, A., Silver, M., van der Zee, R., Li, T., Witzenbichler, B., Schatteman, G., and Isner, J. M., Isolation of putative progenitor endothelial cells for angiogenesis. Science, 275, 964–967 (1997).
Asahara, T. and Kawamoto, A., Endothelial progenitor cells for postnatal vasculogenesis. Am. J. Physiol. Cell Physiol., 287, C572–C579 (2004).
Asai, J., Takenaka, H., Kusano, K. F., Ii, M., Luedemann, C., Curry, C., Eaton, E., Iwakura, A., Tsutsumi, Y., Hamada, H., Kishimoto, S., Thorne, T., Kishore, R., and Losordo, D. W., Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation, 113, 2413–2424 (2006).
Assmus, B., Schachinger, V., Teupe, C., Britten, M., Lehmann, R., Dobert, N., Grunwald, F., Aicher, A., Urbich, C., Martin, H., Hoelzer, D., Dimmeler, S., and Zeiher, A. M., Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (topcare-ami). Circulation, 106, 3009–3017 (2002).
Bahlmann, F. H., de Groot, K., Mueller, O., Hertel, B., Haller, H., and Fliser, D., Stimulation of endothelial progenitor cells: A new putative therapeutic effect of angiotensin II receptor antagonists. Hypertension, 45, 526–529 (2005).
Beckman, J. A., Creager, M. A., and Libby, P., Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA, 287, 2570–2581 (2002).
Brem, H. and Tomic-Canic, M., Cellular and molecular basis of wound healing in diabetes. J. Clin. Invest., 117, 1219–1222 (2007).
Busik, J. V., Tikhonenko, M., Bhatwadekar, A., Opreanu, M., Yakubova, N., Caballero, S., Player, D., Nakagawa, T., Afzal, A., Kielczewski, J., Sochacki, A., Hasty, S., Li Calzi, S., Kim, S., Duclas, S. K., Segal, M. S., Guberski, D. L., Esselman, W. J., Boulton, M. E., and Grant, M. B., Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J. Exp. Med., 206, 2897–2906 (2009).
Butt, E., Abel, K., Krieger, M., Palm, D., Hoppe, V., Hoppe, J., and Walter, U., Camp- and cgmp-dependent protein kinase phosphorylation sites of the focal adhesion vasodilatorstimulated phosphoprotein (vasp) in vitro and in intact human platelets. J. Biol. Chem., 269, 14509–14517 (1994).
Capla, J. M., Grogan, R. H., Callaghan, M. J., Galiano, R. D., Tepper, O. M., Ceradini, D. J., and Gurtner, G. C., Diabetes impairs endothelial progenitor cell-mediated blood vessel formation in response to hypoxia. Plast. Reconstr. Surg., 119, 59–70 (2007).
Ceradini, D. J., Yao, D., Grogan, R. H., Callaghan, M. J., Edelstein, D., Brownlee, M., and Gurtner, G. C., Decreasing intracellular superoxide corrects defective ischemiainduced new vessel formation in diabetic mice. J. Biol. Chem., 283, 10930–10938 (2008).
Chen, J. Z., Zhu, J. H., Wang, X. X., Xie, X. D., Sun, J., Shang, Y. P., Guo, X. G., Dai, H. M., and Hu, S. J., Effects of homocysteine on number and activity of endothelial progenitor cells from peripheral blood. J. Mol. Cell Cardiol., 36, 233–239 (2004).
Chen, S. Y., Wang, F., Yan, X. Y., Zhou, Q., Ling, Q., Ling, J. X., Rong, Y. Z., and Li, Y. G., Autologous transplantation of epcs encoding fgf1 gene promotes neovascularization in a porcine model of chronic myocardial ischemia. Int. J. Cardiol., 135, 223–232 (2009).
Chen, Y. H., Lin, S. J., Lin, F. Y., Wu, T. C., Tsao, C. R., Huang, P. H., Liu, P. L., Chen, Y. L., and Chen, J. W., High glucose impairs early and late endothelial progenitor cells by modi fying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes, 56, 1559–1568 (2007).
Creager, M. A., Luscher, T. F., Cosentino, F., and Beckman, J. A., Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Circulation, 108, 1527–1532 (2003).
Desouza, C. V., Hamel, F. G., Bidasee, K., and O’Connell, K., Role of inflammation and insulin resistance in endothelial progenitor cell dysfunction. Diabetes, 60, 1286–1294 (2011).
Dimmeler, S. and Zeiher, A. M., Endothelial cell apoptosis in angiogenesis and vessel regression. Circ. Res, 87, 434–439 (2000).
Egan, C. G., Lavery, R., Caporali, F., Fondelli, C., Laghi-Pasini, F., Dotta, F., and Sorrentino, V., Generalised reduction of putative endothelial progenitors and CXCR4-positive peripheral blood cells in type 2 diabetes. Diabetologia, 51, 1296–1305 (2008).
Emanueli, C., Monopoli, A., Kraenkel, N., Meloni, M., Gadau, S., Campesi, I., Ongini, E., and Madeddu, P., Nitropravastatin stimulates reparative neovascularisation and improves recovery from limb ischaemia in type-1 diabetic mice. Br. J. Pharmacol., 150, 873–882 (2007).
Endtmann, C., Ebrahimian, T., Czech, T., Arfa, O., Laufs, U., Fritz, M., Wassmann, K., Werner, N., Petoumenos, V., Nickenig, G., and Wassmann, S., Angiotensin II impairs endothelial progenitor cell number and function in vitro and in vivo: Implications for vascular regeneration. Hypertension, 58, 394–403 (2011).
Fadini, G. P., Miorin, M., Facco, M., Bonamico, S., Baesso, I., Grego, F., Menegolo, M., de Kreutzenberg, S. V., Tiengo, A., Agostini, C., and Avogaro, A., Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J. Am. Coll. Cardiol., 45, 1449–1457 (2005).
Fadini, G. P., Coracina, A., Baesso, I., Agostini, C., Tiengo, A., Avogaro, A., and de Kreutzenberg, S. V., Peripheral blood CD34+KDR+ endothelial progenitor cells are determinants of subclinical atherosclerosis in a middle-aged general population. Stroke, 37, 2277–2282 (2006a).
Fadini, G. P., Sartore, S., Albiero, M., Baesso, I., Murphy, E., Menegolo, M., Grego, F., Vigili de Kreutzenberg, S., Tiengo, A., Agostini, C., and Avogaro, A., Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler. Thromb. Vasc. Biol., 26, 2140–2146 (2006b).
Fadini, G. P., Sartore, S., Agostini, C., and Avogaro, A., Significance of endothelial progenitor cells in subjects with diabetes. Diabetes Care, 30, 1305–1313 (2007).
Falanga, V., Wound healing and its impairment in the diabetic foot. Lancet, 366, 1736–1743 (2005).
Fujii, H., Li, S. H., Szmitko, P. E., Fedak, P. W., and Verma, S., C-reactive protein alters antioxidant defenses and promotes apoptosis in endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol., 26, 2476–2482 (2006).
Gallagher, K. A., Liu, Z. J., Xiao, M., Chen, H., Goldstein, L. J., Buerk, D. G., Nedeau, A., Thom, S. R., and Velazquez, O. C., Diabetic impairments in no-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J. Clin. Invest., 117, 1249–1259 (2007).
George, J., Goldstein, E., Abashidze, S., Deutsch, V., Shmilovich, H., Finkelstein, A., Herz, I., Miller, H., and Keren, G., Circulating endothelial progenitor cells in patients with unstable angina: Association with systemic inflammation. Eur. Heart J., 25, 1003–1008 (2004).
Ghani, U., Shuaib, A., Salam, A., Nasir, A., Shuaib, U., Jeerakathil, T., Sher, F., O’Rourke, F., Nasser, A. M., Schwindt, B., and Todd, K., Endothelial progenitor cells during cerebrovascular disease. Stroke, 36, 151–153 (2005).
Guven, H., Shepherd, R. M., Bach, R. G., Capoccia, B. J., and Link, D. C., The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary artery disease. J. Am. Coll. Cardiol., 48, 1579–1587 (2006).
Hamed, S., Brenner, B., Abassi, Z., Aharon, A., Daoud, D., and Roguin, A., Hyperglycemia and oxidized-LDL exert a deleterious effect on endothelial progenitor cell migration in type 2 diabetes mellitus. Thromb. Res., 126, 166–174 (2010).
Hamed, S., Brenner, B., and Roguin, A., Nitric oxide: A key factor behind the dysfunctionality of endothelial progenitor cells in diabetes mellitus type-2. Cardiovasc. Res., 91, 9–15 (2011).
Heeschen, C., Lehmann, R., Honold, J., Assmus, B., Aicher, A., Walter, D. H., Martin, H., Zeiher, A. M., and Dimmeler, S., Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation, 109, 1615–1622 (2004).
Hill, J. M., Zalos, G., Halcox, J. P., Schenke, W. H., Waclawiw, M. A., Quyyumi, A. A., and Finkel, T., Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med., 348, 593–600 (2003).
Holderfield, M. T. and Hughes, C. C., Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ. Res., 102, 637–652 (2008).
Huang, P. H., Huang, S. S., Chen, Y. H., Lin, C. P., Chiang, K. H., Chen, J. S., Tsai, H. Y., Lin, F. Y., Chen, J. W., and Lin, S. J., Increased circulating CD31+/annexin V+ apoptotic microparticles and decreased circulating endothelial progenitor cell levels in hypertensive patients with microalbuminuria. J. Hypertens., 28, 1655–1665 (2010).
Hur, J., Yoon, C. H., Kim, H. S., Choi, J. H., Kang, H. J., Hwang, K. K., Oh, B. H., Lee, M. M., and Park, Y. B., Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler. Thromb. Vasc. Biol., 24, 288–293 (2004)
Ikenaga, S., Hamano, K., Nishida, M., Kobayashi, T., Li, T. S., Kobayashi, S., Matsuzaki, M., Zempo, N., and Esato, K., Autologous bone marrow implantation induced angiogenesis and improved deteriorated exercise capacity in a rat ischemic hindlimb model. J. Surg. Res., 96, 277–283 (2001).
Imanishi, T., Hano, T., Sawamura, T., and Nishio, I., Oxidized low-density lipoprotein induces endothelial progenitor cell senescence, leading to cellular dysfunction. Clin. Exp. Pharmacol. Physiol., 31, 407–413 (2004).
Imanishi, T., Morinobu, A., Hayashi, N., Kanagawa, S., Koshiba, M., Kondo, S., and Kumagai, S., A novel polymorphism of the SSA1 gene is associated with anti-SS-A/ Ro52 autoantibody in japanese patients with primary sjogren’s syndrome. Clin. Exp. Rheumatol., 23, 521–524 (2005).
Irie, H., Tatsumi, T., Takamiya, M., Zen, K., Takahashi, T., Azuma, A., Tateishi, K., Nomura, T., Hayashi, H., Nakajima, N., Okigaki, M., and Matsubara, H., Carbon dioxide-rich water bathing enhances collateral blood flow in ischemic hindlimb via mobilization of endothelial progenitor cells and activation of NO-cGMP system. Circulation, 111, 1523–1529 (2005).
Jarajapu, Y. P. and Grant, M. B., The promise of cell-based therapies for diabetic complications: Challenges and solutions. Circ. Res., 106, 854–869 (2010).
Jujo, K., Hamada, H., Iwakura, A., Thorne, T., Sekiguchi, H., Clarke, T., Ito, A., Misener, S., Tanaka, T., Klyachko, E., Kobayashi, K., Tongers, J., Roncalli, J., Tsurumi, Y., Hagiwara, N., and Losordo, D. W., CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc. Natl. Acad. Sci. U. S. A., 107, 11008–11013 (2010).
Kim, K. L., Meng, Y., Kim, J. Y., Baek, E. J., and Suh, W., Direct and differential effects of stem cell factor on the neovascularization activity of endothelial progenitor cells. Cardiovasc. Res., 92, 132–140 (2011).
Leeper, N. J., Hunter, A. L., and Cooke, J. P., Stem cell therapy for vascular regeneration: Adult, embryonic, and induced pluripotent stem cells. Circulation, 122, 517–526 (2010).
Leone, A. M., Valgimigli, M., Giannico, M. B., Zaccone, V., Perfetti, M., D’Amario, D., Rebuzzi, A. G., and Crea, F., From bone marrow to the arterial wall: The ongoing tale of endothelial progenitor cells. Eur. Heart J., 30, 890–899 (2009).
Leri, A. and Kajstura, J., Endothelial progenitor cells: unexpected disclosures. Circ. Res., 97, 299–301 (2005).
Li Calzi, S., Purich, D. L., Chang, K. H., Afzal, A., Nakagawa, T., Busik, J. V., Agarwal, A., Segal, M. S., and Grant, M. B., Carbon monoxide and nitric oxide mediate cytoskeletal reorganization in microvascular cells via vasodilator-stimulated phosphoprotein phosphorylation: Evidence for blunted responsiveness in diabetes. Diabetes, 57, 2488–2494 (2008).
Liew, A., McDermott, J. H., Barry, F., and O’Brien, T., Endothelial progenitor cells for the treatment of diabetic vasculopathy: Panacea or pandora’s box? Diabetes Obes. Metab., 10, 353–366 (2008).
Lindsay, S. L., Ramsey, S., Aitchison, M., Renne, T., and Evans, T. J., Modulation of lamellipodial structure and dynamics by no-dependent phosphorylation of VASP ser239. J. Cell Sci., 120, 3011–3021 (2007).
Loomans, C. J., de Koning, E. J., Staal, F. J., Rookmaaker, M. B., Verseyden, C., de Boer, H. C., Verhaar, M. C., Braam, B., Rabelink, T. J., and van Zonneveld, A. J., Endothelial progenitor cell dysfunction: A novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes, 53, 195–199 (2004).
Loomans, C. J., De Koning, E. J., Staal, F. J., Rabelink, T. J., and Zonneveld, A. J., Endothelial progenitor cell dysfunction in type 1 diabetes: Another consequence of oxidative stress? Antioxid. Redox Signal., 7, 1468–1475 (2005).
Ma, F. X., Zhou, B., Chen, Z., Ren, Q., Lu, S. H., Sawamura, T., and Han, Z. C., Oxidized low density lipoprotein impairs endothelial progenitor cells by regulation of endothelial nitric oxide synthase. J. Lipid Res., 47, 1227–1237 (2006).
Marrotte, E. J., Chen, D. D., Hakim, J. S., and Chen, A. F., Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. J. Clin. Invest., 120, 4207–4219 (2010).
Moncada, S. and Higgs, E. A., Nitric oxide and the vascular endothelium. Handb. Exp. Pharmacol., 213–254 (2006).
Peichev, M., Naiyer, A. J., Pereira, D., Zhu, Z., Lane, W. J., Williams, M., Oz, M. C., Hicklin, D. J., Witte, L., Moore, M. A., and Rafii, S., Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood, 95, 952–958 (2000).
Peterson, S. J., Husney, D., Kruger, A. L., Olszanecki, R., Ricci, F., Rodella, L. F., Stacchiotti, A., Rezzani, R., McClung, J. A., Aronow, W. S., Ikehara, S., and Abraham, N. G., Longterm treatment with the apolipoprotein a1 mimetic peptide increases antioxidants and vascular repair in type I diabetic rats. J. Pharmacol. Exp. Ther., 322, 514–520 (2007).
Pistrosch, F., Herbrig, K., Oelschlaegel, U., Richter, S., Passauer, J., Fischer, S., and Gross, P., PPARgamma-agonist rosiglitazone increases number and migratory activity of cultured endothelial progenitor cells. Atherosclerosis, 183, 163–167 (2005).
Rabelink, T. J., de Boer, H. C., de Koning, E. J., and van Zonneveld, A. J., Endothelial progenitor cells: More than an inflammatory response? Arterioscler. Thromb. Vasc. Biol., 24, 834–838 (2004).
Reinhard, H., Jacobsen, P. K., Lajer, M., Pedersen, N., Billestrup, N., Mandrup-Poulsen, T., Parving, H. H., and Rossing, P., Multifactorial treatment increases endothelial progenitor cells in patients with type 2 diabetes. Diabetologia, 53, 2129–2133 (2010).
Reyes, M., Dudek, A., Jahagirdar, B., Koodie, L., Marker, P. H., and Verfaillie, C. M., Origin of endothelial progenitors in human postnatal bone marrow. J. Clin. Invest., 109, 337–346 (2002).
Ruiz, E., Redondo, S., Gordillo-Moscoso, A., Rodriguez, E., Reguillo, F., Martinez-Gonzalez, J., and Tejerina, T., Epc adhesion to arteries from diabetic and non-diabetic patients: Effect of pioglitazone. Front. Biosci., 14, 3608–3618 (2009).
Schmidt-Lucke, C., Rossig, L., Fichtlscherer, S., Vasa, M., Britten, M., Kamper, U., Dimmeler, S., and Zeiher, A. M., Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation, 111, 2981–2987 (2005).
Schuster, D. P., Obesity and the development of type 2 diabetes: The effects of fatty tissue inflammation. Diabetes Metab. Syndr. Obes., 3, 253–262 (2010).
Segal, M. S., Shah, R., Afzal, A., Perrault, C. M., Chang, K., Schuler, A., Beem, E., Shaw, L. C., Li Calzi, S., Harrison, J. K., Tran-Son-Tay, R., and Grant, M. B., Nitric oxide cytoskeletal- induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes, 55, 102–109 (2006).
Shantsila, E., Watson, T., and Lip, G. Y., Endothelial progenitor cells in cardiovascular disorders. J. Am. Coll. Cardiol., 49, 741–752 (2007).
Shantsila, E., Watson, T., Tse, H. F., and Lip, G. Y., New insights on endothelial progenitor cell subpopulations and their angiogenic properties. J. Am. Coll. Cardiol., 51, 669–671 (2008).
Shi, Q., Rafii, S., Wu, M. H., Wijelath, E. S., Yu, C., Ishida, A., Fujita, Y., Kothari, S., Mohle, R., Sauvage, L. R., Moore, M. A., Storb, R. F., and Hammond, W. P., Evidence for circulating bone marrow-derived endothelial cells. Blood, 92, 362–367 (1998).
Shimada, K., Mokuno, H., Matsunaga, E., Miyazaki, T., Sumiyoshi, K., Kume, A., Miyauchi, K., and Daida, H., Predictive value of circulating oxidized LDL for cardiac events in type 2 diabetic patients with coronary artery disease. Diabetes Care, 27, 843–844 (2004).
Sorrentino, S. A., Bahlmann, F. H., Besler, C., Muller, M., Schulz, S., Kirchhoff, N., Doerries, C., Horvath, T., Limbourg, A., Limbourg, F., Fliser, D., Haller, H., Drexler, H., and Landmesser, U., Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: Restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation, 116, 163–173 (2007).
Strauer, B. E., Brehm, M., Zeus, T., Kostering, M., Hernandez, A., Sorg, R. V., Kogler, G., and Wernet, P., Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation, 106, 1913–1918 (2002).
Taguchi, A., Matsuyama, T., Moriwaki, H., Hayashi, T., Hayashida, K., Nagatsuka, K., Todo, K., Mori, K., Stern, D. M., Soma, T., and Naritomi, H., Circulating CD34-positive cells provide an index of cerebrovascular function. Circulation, 109, 2972–2975 (2004).
Takahashi, K. and Yamanaka, S., Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126, 663–676 (2006).
Tepper, O. M., Galiano, R. D., Capla, J. M., Kalka, C., Gagne, P. J., Jacobowitz, G. R., Levine, J. P., and Gurtner, G. C., Human endothelial progenitor cells from type ii diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation, 106, 2781–2786 (2002).
Thum, T., Fleissner, F., Klink, I., Tsikas, D., Jakob, M., Bauersachs, J., and Stichtenoth, D. O., Growth hormone treatment improves markers of systemic nitric oxide bioavailability via insulin-like growth factor-i. J. Clin. Endocrinol. Metab., 92, 4172–4179 (2007a).
Thum, T., Fraccarollo, D., Schultheiss, M., Froese, S., Galuppo, P., Widder, J. D., Tsikas, D., Ertl, G., and Bauersachs, J., Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes, 56, 666–674 (2007b).
Tian, F., Liang, P. H., and Li, L. Y., Inhibition of endothelial progenitor cell differentiation by vegi. Blood, 113, 5352–5360 (2009).
Tie, G., Yan, J., Yang, Y., Park, B. D., Messina, J. A., Raffai, R. L., Nowicki, P. T., and Messina, L. M., Oxidized lowdensity lipoprotein induces apoptosis in endothelial progenitor cells by inactivating the phosphoinositide 3-kinase/ akt pathway. J. Vasc. Res., 47, 519–530 (2010).
Tilki, D., Hohn, H. P., Ergun, B., Rafii, S., and Ergun, S., Emerging biology of vascular wall progenitor cells in health and disease. Trends Mol. Med., 15, 501–509 (2009).
Togliatto, G., Trombetta, A., Dentelli, P., Baragli, A., Rosso, A., Granata, R., Ghigo, D., Pegoraro, L., Ghigo, E., and Brizzi, M. F., Unacylated ghrelin rescues endothelial progenitor cell function in individuals with type 2 diabetes. Diabetes, 59, 1016–1025 (2010).
Tousoulis, D., Andreou, I., Antoniades, C., Tentolouris, C., and Stefanadis, C., Role of inflammation and oxidative stress in endothelial progenitor cell function and mobilization: Therapeutic implications for cardiovascular diseases. Atherosclerosis, 201, 236–247 (2008).
Tse, H. F., Thambar, S., Kwong, Y. L., Rowlings, P., Bellamy, G., McCrohon, J., Thomas, P., Bastian, B., Chan, J. K., Lo, G., Ho, C. L., Chan, W. S., Kwong, R. Y., Parker, A., Hauser, T. H., Chan, J., Fong, D. Y., and Lau, C. P., Prospective randomized trial of direct endomyocardial implantation of bone marrow cells for treatment of severe coronary artery diseases (protect-cad trial). Eur. Heart J., 28, 2998–3005 (2007).
Urbich, C. and Dimmeler, S., Endothelial progenitor cells: Characterization and role in vascular biology. Circ. Res., 95, 343–353 (2004).
Valgimigli, M., Rigolin, G. M., Fucili, A., Porta, M. D., Soukhomovskaia, O., Malagutti, P., Bugli, A. M., Bragotti, L. Z., Francolini, G., Mauro, E., Castoldi, G., and Ferrari, R., CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation, 110, 1209–1212 (2004).
Vasa, M., Fichtlscherer, S., Aicher, A., Adler, K., Urbich, C., Martin, H., Zeiher, A. M., and Dimmeler, S., Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ. Res., 89, E1–E7 (2001).
Verma, S. and Anderson, T. J., Fundamentals of endothelial function for the clinical cardiologist. Circulation, 105, 546–549 (2002).
Werner, C., Kamani, C. H., Gensch, C., Bohm, M., and Laufs, U., The peroxisome proliferator-activated receptor-gamma agonist pioglitazone increases number and function of endothelial progenitor cells in patients with coronary artery disease and normal glucose tolerance. Diabetes, 56, 2609–2615 (2007).
Werner, N., Kosiol, S., Schiegl, T., Ahlers, P., Walenta, K., Link, A., Bohm, M., and Nickenig, G., Circulating endothelial progenitor cells and cardiovascular outcomes. N. Engl. J. Med., 353, 999–1007 (2005).
Westerweel, P. E. and Verhaar, M. C., Endothelial progenitor cell dysfunction in rheumatic disease. Nat. Rev. Rheumatol., 5, 332–340 (2009).
Wu, Y., Wang, Q., Cheng, L., Wang, J., and Lu, G., Effect of oxidized low-density lipoprotein on survival and function of endothelial progenitor cell mediated by p38 signal pathway. J. Cardiovasc. Pharmacol., 53, 151–156 (2009).
Xiao, Q., Kiechl, S., Patel, S., Oberhollenzer, F., Weger, S., Mayr, A., Metzler, B., Reindl, M., Hu, Y., Willeit, J., and Xu, Q., Endothelial progenitor cells, cardiovascular risk factors, cytokine levels and atherosclerosis—results from a large population-based study. PLoS ONE, 2, e975 (2007).
Young, H. E., Duplaa, C., Katz, R., Thompson, T., Hawkins, K. C., Boev, A. N., Henson, N. L., Heaton, M., Sood, R., Ashley, D., Stout, C., Morgan, J. H., 3rd, Uchakin, P. N., Rimando, M., Long, G. F., Thomas, C., Yoon, J. I., Park, J. E., Hunt, D. J., Walsh, N. M., Davis, J. C., Lightner, J. E., Hutchings, A. M., Murphy, M. L., Boswell, E., McAbee, J. A., Gray, B. M., Piskurich, J., Blake, L., Collins, J. A., Moreau, C., Hixson, D., Bowyer, F. P., 3rd, and Black, A. C., Jr., Adult-derived stem cells and their potential for use in tissue repair and molecular medicine. J. Cell. Mol. Med., 9, 753–769 (2005).
Zhang, Y., Ingram, D. A., Murphy, M. P., Saadatzadeh, M. R., Mead, L. E., Prater, D. N., and Rehman, J., Release of proinflammatory mediators and expression of proinflammatory adhesion molecules by endothelial progenitor cells. Am. J. Physiol. Heart Circ. Physiol., 296, H1675–H1682 (2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, KA., Shin, YJ., Kim, JH. et al. Dysfunction of endothelial progenitor cells under diabetic conditions and its underlying mechanisms. Arch. Pharm. Res. 35, 223–234 (2012). https://doi.org/10.1007/s12272-012-0203-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-012-0203-y