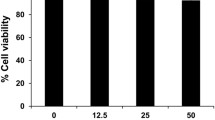
Abstract
Transforming growth factor-β1 (TGFβ1) induces epithelial-to-mesenchymal transition (EMT) in cultured renal tubular epithelial cells. This phenotypic transition has been known to be involved in the development of chronic kidney diseases by activating profibrotic gene expression. Since oxidative stress has been recognized as one of the contributors to this TGFβ1-mediated pathology, we investigated the potential involvement of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), which is a key transcription factor for the regulation of multiple antioxidant genes, in TGFβ1-stimulated EMT gene changes using the rat proximal tubular epithelial cell line NRK52E. The treatment of NRK52E with TGFβ1 led to changes in EMT gene expression, including increased α-Sma and decreased E-cadherin expression. In these cells, the TGFβ1 treatment decreased the transcript level of the catalytic subunit of γ-glutamate cysteine ligase (Gclc), a glutathione (GSH) biosynthetic enzyme, and reduced the total GSH content with a concomitant decrease in Nrf2 transcription activity. Accordantly, pre-incubation with the GSH precursor N-acetylcysteine attenuated TGFβ1-stimulated EMT gene changes. The involvement of Nrf2 in EMT gene changes has been demonstrated using NRK52E cells with nrf2 knockdown or pharmacological activation. When the expression of Nrf2 was stably silenced in NRK52E cells using interfering RNA administration, Gclc expression was significantly reduced and the increase in the levels of α-Sma and fibronectin-1 by TGFβ1 was greater than those in the nonspecific RNA control group. Conversely, Nrf2 activation and subsequent Gclc increase by Nrf2-activating sulforaphane alleviated the TGFβ1-stimulated α-Sma increase and E-cadherin decrease. Collectively, these results indicate that Nrf2-GSH signaling can modulate TGFβ1-stimulated EMT gene changes and further suggest a beneficial role of Nrf2 inducers in renal pathogenesis.






Similar content being viewed by others
Abbreviations
- TGFβ1:
-
Transforming growth factor-β1
- EMT:
-
Epithelial-to-mesenchymal transition
- Nrf2i:
-
Nrf2 knockdown NRK52E
- sc:
-
Nonspecific scrambled RNA NRK52E
- ARE:
-
Antioxidant-response element
- ROS:
-
Reactive oxygen species
- GSH:
-
Glutathione
- Gclc:
-
Catalytic subunit of γ-glutamate cysteine ligase
- Ho-1:
-
Heme oxygenase-1
- α-Sma:
-
α-smooth muscle actin
- Fn-1:
-
Fibronectin-1
- Col1A1:
-
Collagen 1A1
- Cdh1:
-
E-cadherin
- Hprt:
-
Hypoxanthine–guanine phosphoribosyltransferase
- SFN:
-
Sulforaphane
References
Artaud-Macari, E., D. Goven, S. Brayer, A. Hamimi, V. Besnard, J. Marchal-Somme, Z.E. Ali, B. Crestani, S. Kerdine-Romer, A. Boutten, and M. Bonay. 2013. Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxidants and Redox Signaling 18: 66–79.
Bakin, A.V., N.V. Stourman, K.R. Sekhar, C. Rinehart, X. Yan, M.J. Meredith, C.L. Arteaga, and M.L. Freeman. 2005. Smad3-ATF3 signaling mediates TGF-beta suppression of genes encoding Phase II detoxifying proteins. Free Radical Biology and Medicine 38: 375–387.
Biernacka, A., M. Dobaczewski, and N.G. Frangogiannis. 2011. TGF-beta signaling in fibrosis. Growth Factors 29: 196–202.
Bondi, C.D., N. Manickam, D.Y. Lee, K. Block, Y. Gorin, H.E. Abboud, and J.L. Barnes. 2010. NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. Journal of the American Society of Nephrology 21: 93–102.
Brown, S.L., K.R. Sekhar, G. Rachakonda, S. Sasi, and M.L. Freeman. 2008. Activating transcription factor 3 is a novel repressor of the nuclear factor erythroid-derived 2-related factor 2 (Nrf2)-regulated stress pathway. Cancer Research 68: 364–368.
Choi, H.K., Pokharel, Y.R., Lim, S.C., Han, H.K., Ryu, C.S., Kim, S.K., Kwak, M.K., and Kang, K.W. 2009. Inhibition of liver fibrosis by solubilized coenzyme Q10: Role of Nrf2 activation in inhibiting transforming growth factor-beta1 expression. Toxicology and Applied Pharmacology.
Derynck, R., and Y.E. Zhang. 2003. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425: 577–584.
Fragiadaki, M., and R.M. Mason. 2011. Epithelial–mesenchymal transition in renal fibrosis: Evidence for and against. International Journal of Experimental Pathology 92: 143–150.
Franklin, C.C., M.E. Rosenfeld-Franklin, C. White, T.J. Kavanagh, and N. Fausto. 2003. TGFbeta1-induced suppression of glutathione antioxidant defenses in hepatocytes: caspase-dependent post-translational and caspase-independent transcriptional regulatory mechanisms. Faseb J 17: 1535–1537.
Hackett, T.L., S.M. Warner, D. Stefanowicz, F. Shaheen, D.V. Pechkovsky, L.A. Murray, R. Argentieri, A. Kicic, S.M. Stick, T.R. Bai, and D.A. Knight. 2009. Induction of epithelial–mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. American Journal of Respiratory and Critical Care Medicine 180: 122–133.
Hertig, A., J. Verine, B. Mougenot, C. Jouanneau, N. Ouali, P. Sebe, D. Glotz, P.Y. Ancel, E. Rondeau, and Y.C. Xu-Dubois. 2006. Risk factors for early epithelial to mesenchymal transition in renal grafts. American Journal of Transplantation 6: 2937–2946.
Hwang, M., H.J. Kim, H.J. Noh, Y.C. Chang, Y.M. Chae, K.H. Kim, J.P. Jeon, T.S. Lee, H.K. Oh, Y.S. Lee, and K.K. Park. 2006. TGF-beta1 siRNA suppresses the tubulointerstitial fibrosis in the kidney of ureteral obstruction. Experimental and Molecular Pathology 81: 48–54.
Itoh, K., N. Wakabayashi, Y. Katoh, T. Ishii, K. Igarashi, J.D. Engel, and M. Yamamoto. 1999. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes and Development 13: 76–86.
Iwano, M., D. Plieth, T.M. Danoff, C. Xue, H. Okada, and E.G. Neilson. 2002. Evidence that fibroblasts derive from epithelium during tissue fibrosis. Journal of Clinical Investigation 110: 341–350.
Jiang, T., Z. Huang, Y. Lin, Z. Zhang, D. Fang, and D.D. Zhang. 2010. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes 59: 850–860.
Jiang, Y.S., T. Jiang, B. Huang, P.S. Chen, and J. Ouyang. 2013. Epithelial–mesenchymal transition of renal tubules: Divergent processes of repairing in acute or chronic injury? Medical Hypotheses 81: 73–75.
Kalluri, R., and R.A. Weinberg. 2009. The basics of epithelial–mesenchymal transition. Journal of Clinical Investigation 119: 1420–1428.
Kasai, H., J.T. Allen, R.M. Mason, T. Kamimura, and Z. Zhang. 2005. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respiratory Research 6: 56.
Kashihara, N., Y. Haruna, V.K. Kondeti, and Y.S. Kanwar. 2010. Oxidative stress in diabetic nephropathy. Current Medicinal Chemistry 17: 4256–4269.
Kobayashi, A., M.I. Kang, H. Okawa, M. Ohtsuji, Y. Zenke, T. Chiba, K. Igarashi, and M. Yamamoto. 2004. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Molecular and Cellular Biology 24: 7130–7139.
Kobayashi, M., and M. Yamamoto. 2005. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxidants and Redox Signaling 7: 385–394.
Kwak, M.K., and T.W. Kensler. 2010. Targeting NRF2 signaling for cancer chemoprevention. Toxicology and Applied Pharmacology 244: 66–76.
Kwak, M.K., N. Wakabayashi, K. Itoh, H. Motohashi, M. Yamamoto, and T.W. Kensler. 2003. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway: Identification of novel gene clusters for cell survival. Journal of Biological Chemistry 278: 8135–8145.
Lee, J.M., S. Dedhar, R. Kalluri, and E.W. Thompson. 2006. The epithelial–mesenchymal transition: new insights in signaling, development, and disease. Journal of Cell Biology 172: 973–981.
Lewerenz, J., and P. Maher. 2011. Control of redox state and redox signaling by neural antioxidant systems. Antioxidants and Redox Signaling 14: 1449–1465.
Li, W., and A.N. Kong. 2009. Molecular mechanisms of Nrf2-mediated antioxidant response. Molecular Carcinogenesis 48: 91–104.
Liu, R.M., and K.A. Gaston Pravia. 2010. Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Radical Biology and Medicine 48: 1–15.
Liu, R.M., P.K. Vayalil, C. Ballinger, D.A. Dickinson, W.T. Huang, S. Wang, T.J. Kavanagh, Q.L. Matthews, and E.M. Postlethwait. 2012. Transforming growth factor beta suppresses glutamate-cysteine ligase gene expression and induces oxidative stress in a lung fibrosis model. Free Radical Biology and Medicine 53: 554–563.
Liu, Y. 2004. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. Journal of the American Society of Nephrology 15: 1–12.
Margetts, P.J., P. Bonniaud, L. Liu, C.M. Hoff, C.J. Holmes, J.A. West-Mays, and M.M. Kelly. 2005. Transient overexpression of TGF-β1 induces epithelial mesenchymal transition in the rodent peritoneum. Journal of the American Society of Nephrology 16: 425–436.
Nakamura, T., M. Matsushima, Y. Hayashi, M. Shibasaki, K. Imaizumi, N. Hashimoto, K. Shimokata, Y. Hasegawa, and T. Kawabe. 2011. Attenuation of transforming growth factor-beta-stimulated collagen production in fibroblasts by quercetin-induced heme oxygenase-1. American Journal of Respiratory Cell and Molecular Biology 44: 614–620.
Oh, C.J., J.Y. Kim, A.K. Min, K.G. Park, R.A. Harris, H.J. Kim, and I.K. Lee. 2012. Sulforaphane attenuates hepatic fibrosis via NF-E2-related factor 2-mediated inhibition of transforming growth factor-beta/Smad signaling. Free Radical Biology and Medicine 52: 671–682.
Paine, A., B. Eiz-Vesper, R. Blasczyk, and S. Immenschuh. 2010. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochemical Pharmacology 80: 1895–1903.
Pat, B., T. Yang, C. Kong, D. Watters, D.W. Johnson, and G. Gobe. 2005. Activation of ERK in renal fibrosis after unilateral ureteral obstruction: modulation by antioxidants. Kidney International 67: 931–943.
Pohlers, D., J. Brenmoehl, I. Loffler, C.K. Muller, C. Leipner, S. Schultze-Mosgau, A. Stallmach, R.W. Kinne, and G. Wolf. 2009. TGF-beta and fibrosis in different organs—molecular pathway imprints. Biochimica et Biophysica Acta 1792: 746–756.
Rastaldi, M.P., F. Ferrario, L. Giardino, G. Dell’antonio, C. Grillo, P. Grillo, F. Strutz, G.A. Muller, G. Colasanti, and G. D’amico. 2002. Epithelial–mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney International 62: 137–146.
Rhyu, D.Y., Y. Yang, H. Ha, G.T. Lee, J.S. Song, S.T. Uh, and H.B. Lee. 2005. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial–mesenchymal transition in renal tubular epithelial cells. Journal of the American Society of Nephrology 16: 667–675.
Sato, M., Y. Muragaki, S. Saika, A.B. Roberts, and A. Ooshima. 2003. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. Journal of Clinical Investigation 112: 1486–1494.
Shin, D.H., H.M. Park, K.A. Jung, H.G. Choi, J.A. Kim, D.D. Kim, S.G. Kim, K.W. Kang, S.K. Ku, T.W. Kensler, and M.K. Kwak. 2010. The NRF2-heme oxygenase-1 system modulates cyclosporin A-induced epithelial–mesenchymal transition and renal fibrosis. Free Radical Biology and Medicine 48: 1051–1063.
Simonson, M.S. 2007. Phenotypic transitions and fibrosis in diabetic nephropathy. Kidney International 71: 846–854.
Soetikno, V., F.R. Sari, A.P. Lakshmanan, S. Arumugam, M. Harima, K. Suzuki, H. Kawachi, and K. Watanabe. 2013. Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2-keap1 pathway. Molecular Nutrition and Food Research 57: 1649–1659.
Wakabayashi, N., A.T. Dinkova-Kostova, W.D. Holtzclaw, M.L. Kang, A. Kobayashi, M. Yamamoto, T.W. Kensler, and P. Talalay. 2004. Protection against electrophile and oxidant stress by induction of the phase 2 response: Fate of cysteines of the Keap1 sensor modified by inducers. Proceedings of the National Academy of Sciences of the United States of America 101: 2040–2045.
Willis, B.C., J.M. Liebler, K. Luby-Phelps, A.G. Nicholson, E.D. Crandall, R.M. Du Bois, and Z. Borok. 2005. Induction of epithelial–mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: Potential role in idiopathic pulmonary fibrosis. American Journal of Pathology 166: 1321–1332.
Wynn, T.A. 2008. Cellular and molecular mechanisms of fibrosis. Journal of Pathology 214: 199–210.
Yamamoto, T., T. Suzuki, A. Kobayashi, J. Wakabayashi, J. Maher, H. Motohashi, and M. Yamamoto. 2008. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Molecular and Cellular Biology 28: 2758–2770.
Zarjou, A., and A. Agarwal. 2012. Heme oxygenase-1 as a target for TGF-beta in kidney disease. Seminars in Nephrology 32: 277–286.
Zavadil, J., and E.P. Bottinger. 2005. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 24: 5764–5774.
Zhang, D.D., S.C. Lo, J.V. Cross, D.J. Templeton, and M. Hannink. 2004. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Molecular and Cellular Biology 24: 10941–10953.
Zhang, M., Z. Zhang, H.Y. Pan, D.X. Wang, Z.T. Deng, and X.L. Ye. 2009. TGF-beta1 induces human bronchial epithelial cell-to-mesenchymal transition in vitro. Lung 187: 187–194.
Acknowledgments
This research was supported by the Bio and Medical Technology Development Program (NRF-2013M3A9B5075839) of the National Research Foundation (NRF) funded by the Ministry of Science, ICT and Future Planning, and the Basic Science Research Program (2011-0003619) of the NRF funded by the Ministry of Education, Science and Technology.
Conflict of interest
We have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ryoo, Ig., Shin, Dh., Kang, KS. et al. Involvement of Nrf2-GSH signaling in TGFβ1-stimulated epithelial-to-mesenchymal transition changes in rat renal tubular cells. Arch. Pharm. Res. 38, 272–281 (2015). https://doi.org/10.1007/s12272-014-0380-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-014-0380-y